Technology Empowers Healthcare: Clover Medical’s Handheld Robotic-Assisted Surgical System Sets Sail Globally
Recently, Clover Medical successfully performed the first overseas handheld robotic-assisted hip and knee replacement surgeries in Turkey, marking the official debut of China's independently developed orthopedic surgical robot technology on the global stage. This milestone achievement not only demonstrates Clover Medical's leading strength in the field of smart orthopedics but also brings more precise and safer treatment options to joint disease patients worldwide.
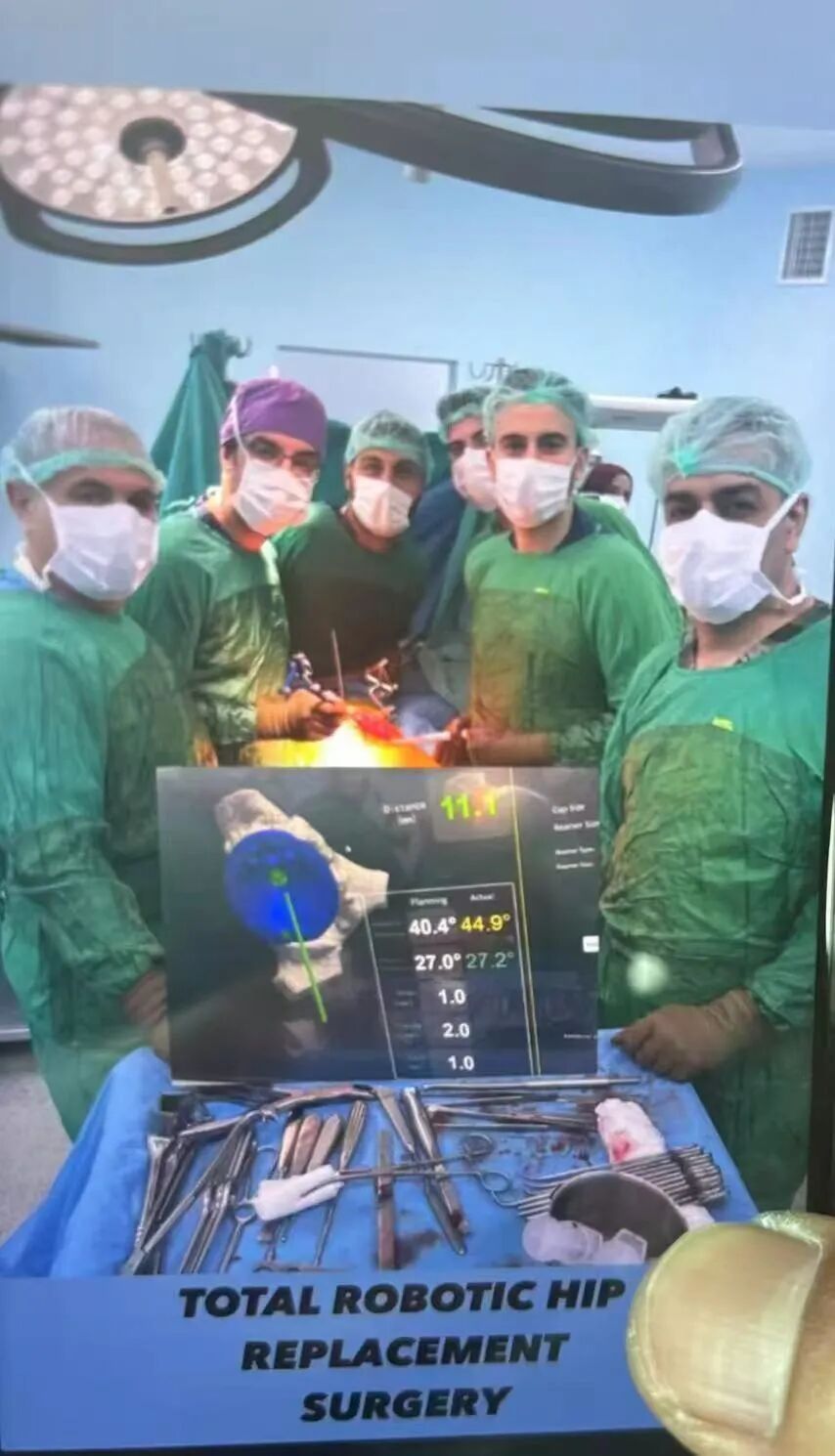
The surgery was a complete success, with precision reaching sub-millimeter level.
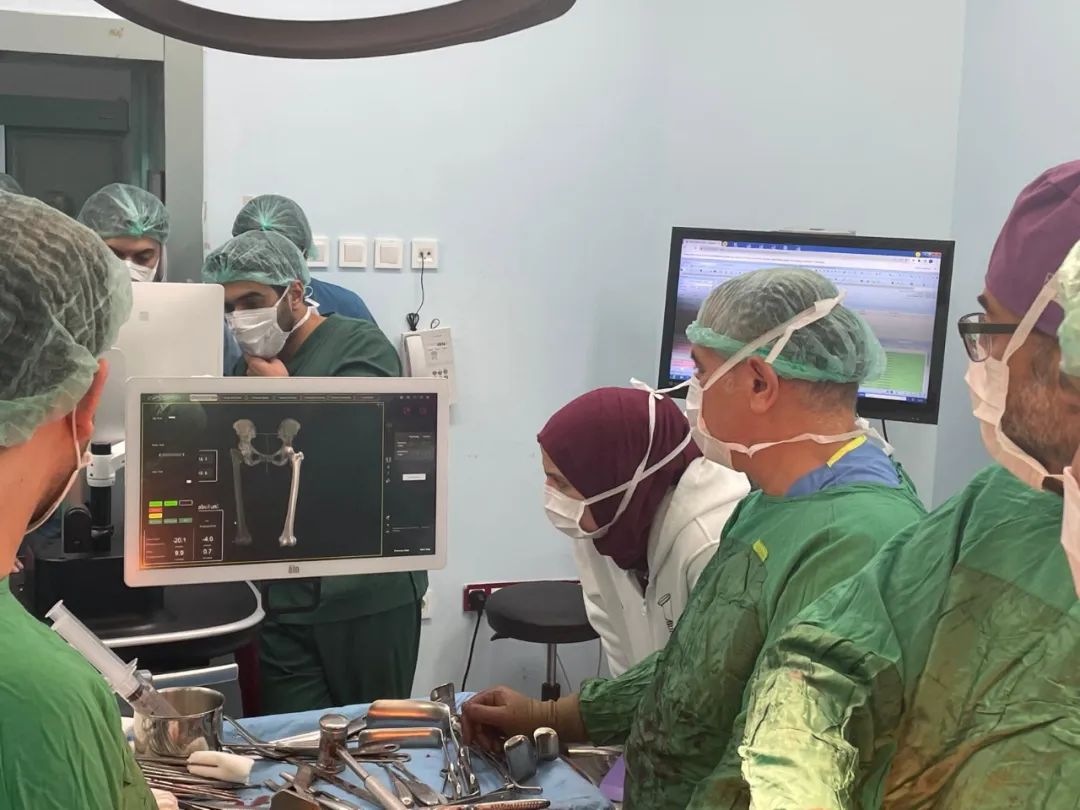
The surgeries were performed at Eğitim Araştırma Hastanesi, Gülhane Eğitim Araştırma Hastanesi and Kırıkkale Üniversitesi Tıp Fakültesi Hastanesi in Ankara, the capital city of Turkey. Led by the renowned orthopedic surgeon Ugur Tiftikci and his team, a total of 4 hip replacement surgeries and 2 knee replacement surgeries were successfully completed, utilizing Clover Medical’s independently developed Yangtze INS-1 Handheld Robotic System for Hip Replacement and Yellow River INS-1 Handheld Robotic System for Knee Replacement. Through preoperative intelligent planning, intraoperative real-time navigation and precise safety boundary control, the procedures achieved exceptional accuracy with a prosthetic implantation angular error of less than 1° and positional deviation of less than 1 mm, far exceeding the precision level of traditional surgeries.
"Robotic-assisted technology allows us to perform hip and knee replacement surgeries with unprecedented precision," stated the lead surgeon postoperatively. He added that the patients exhibited significant reduction in blood loss, were able to get out of bed and ambulate on the second day after surgery, and their recovery speed was truly remarkable.
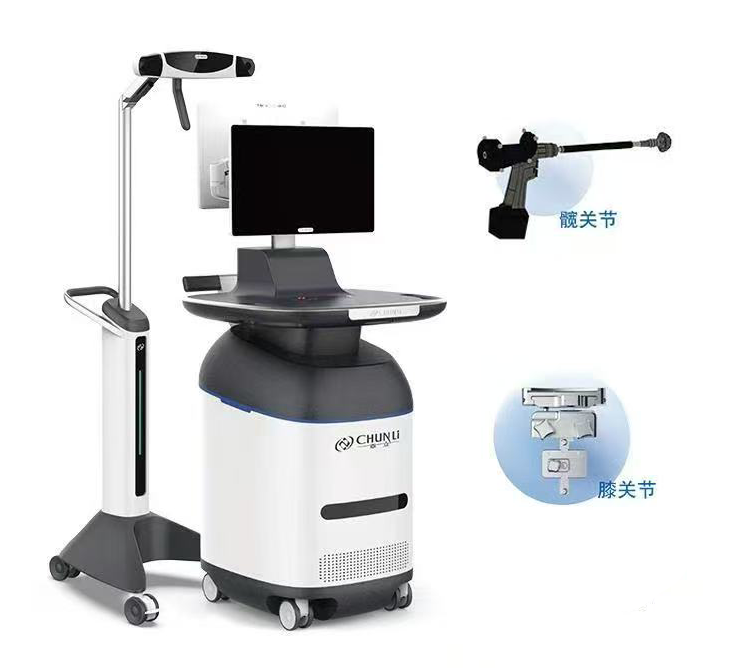
Chunli Medical: From Made in China to Shared Globally
As a leading enterprise in China’s joint prosthesis sector, Clover Medical has always adhered to an innovation-driven development strategy. The orthopedic surgical robotic systems developed by the company integrate cutting-edge technologies such as artificial intelligence, computer vision, and precision control, with abundant successful cases accumulated domestically.
This debut in Turkey marks a crucial step in Clover Medical’s internationalization strategy. We aim to enable global patients to access high-quality medical services brought by "Intelligent Manufacturing in China" through technological innovation. The first case in Turkey is just the starting point; in the future, the company will promote this advanced technology in more countries and regions.
The New Era of Intelligent Orthopedics: More Precise, Less Invasive, and Safer
Traditional joint replacement surgery is highly dependent on surgeons' experience, with issues such as a long learning curve and unavoidable operational errors. Through CT segmentation modeling, intelligent planning, and precise execution, the robotic-assisted system significantly enhances the predictability and repeatability of surgeries.Clinical data i
Clinical data indicates that robot-navigated assisted surgery can reduce soft tissue damage by over 30%, lower the risk of dislocation by 50%, and extend the expected service life of prostheses by 30%-50%. These advantages are particularly crucial for young patients with high activity demands.
Looking Ahead: Chunli Medical Accelerates the Global Layout of Its Smart Orthopedic Ecosystem
Following the success of its first overseas surgical procedure, Clover Medical plans to establish multiple robotic-assisted surgery training centers overseas within the next three years, cultivating a pool of international professionals specializing in smart orthopedics. Meanwhile, adhering to the R&D and production philosophy of "Developing and Manufacturing Products as if for Personal Use, with Continuous Innovation", the company will keep advancing technology iterations to ensure the safety and efficacy of its products in clinical applications, thereby better serving orthopedic patients suffering from pain across China and around the world.
We are convinced that smart orthopedic technology will reshape the standards of joint replacement surgery, and Chinese innovation will play an increasingly important role in this transformation.
The success of this surgical procedure in Turkey not only brings benefits to local patients, but also demonstrates to the world the technological innovation capabilities of Chinese medical device enterprises. With its innovative "robotics + orthopedics" model, Clover Medical is driving global joint surgery into a new era of intelligence.
We sincerely invite you to follow the latest developments of Chunli Medical and witness how Chunli Medical's orthopedic surgical robots use advanced technology to improve human health.
Company Introduction
Clover Medical Technology Co., Ltd. (688236.SH; 01858.HK) was founded in 1998. Since its establishment, the company has been committed to advancing the continuous development and research of high-end medical devices. Its product portfolio covers a full range of orthopedic devices, including joint, spinal, sports medicine and trauma products. Meanwhile, the company is actively expanding into such fields as dentistry, platelet-rich plasma (PRP) preparation systems and orthopedic surgical robots to continuously broaden its product lines.
Clover Medical holds 158 medical device registration certificates and filing documents, covering multiple product categories including joints, spine, sports medicine, trauma, dentistry and PRP. The acquisition of multi-category registration certificates has further enriched the company’s product portfolio, expanded its footprint in the medical field, continuously met diversified market and clinical needs, and helped enhance its overall competitiveness. While maintaining its leading position in the domestic market, the company is also actively exploring the international market. At present, its products have been exported to more than 50 countries and regions worldwide.
With over two decades of development, Chunli Medical boasts a workforce of more than 1,000 employees, including professional and technical talents such as postdoctoral researchers, PhD holders, MBA graduates, master’s degree holders, bachelor’s degree holders, and industry veterans with 20-plus years of hands-on experience. The company has built a multidisciplinary and composite professional team covering mechanical design, materials science, biomechanics, clinical medicine, computer science and other fields.As one of the G20 Innovation-Leading Enterprises during the 13th Five-Year Plan period, Chunli Medical holds qualifications including National High-Tech Enterprise, Beijing Artificial Joint Engineering Laboratory and Beijing Enterprise Technology Center, and operates research platforms such as the Postdoctoral Research Workstation. It has undertaken projects under the Ministry of Science and Technology Talent Program, the National Natural Science Foundation of China and the China Postdoctoral Science Foundation, as well as multiple government-sponsored research projects initiated by the Beijing Municipal Science and Technology Commission, the Beijing Municipal Development and Reform Commission, and the Beijing Municipal Bureau of Economy and Information Technology. In 2023, the company was accredited as a National Manufacturing Single Champion Enterprise, and three of its products—Degradable Medical Magnesium Alloy Materials, Tantalum Powder for Medical Additive Manufacturing and Biphasic Calcium Phosphate—were simultaneously selected into the first batch of the National Biomedical Materials Innovation Task Force under the "Challenge Program".A leading player in China’s domestic joint replacement industry, Chunli Medical has secured unique advantages and leading market positions with a diverse range of products. These offerings have filled critical market gaps, strengthened the company’s core competitiveness and industry recognition, and elevated the level of independent innovation in China’s joint replacement sector.
As a leader in China’s domestic joint device industry, Clover Medical will continue to uphold the philosophy of "Absorbing the Essence of Predecessors and Developing High-quality Products for the World", and adhere to the principle of "Developing and Manufacturing Products as if for Personal Use, with Continuous Innovation" for product R&D and manufacturing. It is committed to providing high-quality products to global customers and emerging as a world-renowned orthopedic enterprise.
>Beijing Chunlizhengda Medical Devices Co., Ltd.
>No. 10, Xinmi West 2nd Road, Tongzhou District, Beijing
>www.clzd.com
>Beijing Medical Device Registration (Document) No. 290515-20068
>National Medical Device Registration Number: 20243010921



