
Domestic joint technology reaches new heights!
April 2025,Chunli Medical's independently developed 'biological total knee prosthesis' has been officially approved for market launch (Registration Certificate No.: Guo Xie Zhu Zhun 20253130823)!This product is the country's first 'dual-coated' biologic total knee prosthesis, once again leveraging technological innovation to bring a better solution for patients suffering from knee joint problems!
Why choose a biologic total knee prosthesis?
With the rising global incidence of osteoarthritis and rheumatoid arthritis and the intensification of population aging, an increasing number of people are more receptive to knee replacement surgery. In recent years, technological advancements and the upgrading of patient demands have been driving the rapid development of cementless knee prosthesis technology. The collaborative innovation of new materials, manufacturing processes and precision surgical techniques has significantly enhanced the stability and durability of implants, garnered widespread clinical recognition, and further stimulated the sustained growth of the market size.
According to relevant reports, the total market size of cementless prostheses in the United States reached approximately 1.8 billion US dollars in 2024. It is projected that the compound annual growth rate (CAGR) will stand at around 5.8% over the next decade, and such technology will gradually emerge as a pivotal development direction in the field of knee replacement.
Biological integration, saying goodbye to 'cement dependency'
Traditional cemented prostheses achieve fixation via a chemical bonding mechanism (the cement-bone interface). While this represents the conventional fixation method for knee replacement, long-term use may trigger risks such as osteolysis and implant loosening, and it fails to meet the higher requirements for prosthesis longevity among younger patients.
The composite coating of Clover’s biotype total knee prosthesis adopts vacuum plasma spraying (VPS) of pure titanium combined with atmospheric plasma spraying (APS) of hydroxyapatite (HA). As a bioactive material, hydroxyapatite (HA) can form direct chemical bonds with bone tissue (osseointegration). Thanks to this property, the composite coating acts as a scaffold for new bone growth, fostering a strong and stable interface between the implant and host tissue. Meanwhile, the bioactive coating mimics the structure of natural bone trabeculae; guided by HA, bone tissue grows into the porous structure to achieve biological fixation. The pure titanium coating reinforced with HA maximizes the performance of the bioactive coating, thereby reducing the risk of postoperative implant loosening and enhancing long-term implant stability. This technology not only effectively promotes tight integration between bone tissue and the implant, but also significantly lowers the revision risk caused by cement aging, making it particularly suitable for younger patients with high activity levels.
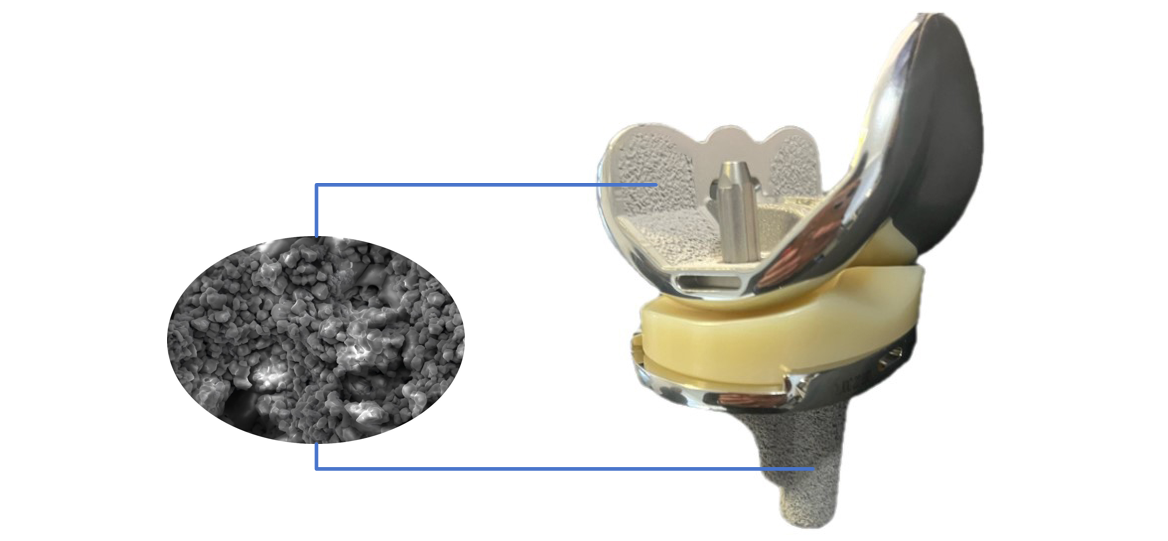
Optimized design, adaptable to diverse needs
①Femoral condyle:
★ Gradual radius design enhances the conformity between the femoral condyles and the liner, avoiding instability in the mid-flexion phase.
★ Thinned anterior condyle and optimized patellar trochlea design reduce patellar crepitus and anterior knee pain.
★ Optimized open intercondylar box design minimizes the amount of intercondylar osteotomy.
★ Provide PS/CR type prostheses.

②Tibial plateau support:
★ Anatomical structure design enables maximum coverage of the tibial osteotomy surface, ensuring uniform pressure distribution on the proximal tibia and reducing irritation of surrounding soft tissues by the tibial tray.
★ Highly polished design on the upper surface of the tibial plateau tray can minimize wear between the underside of the liner and the contact surface of the tibial tray.

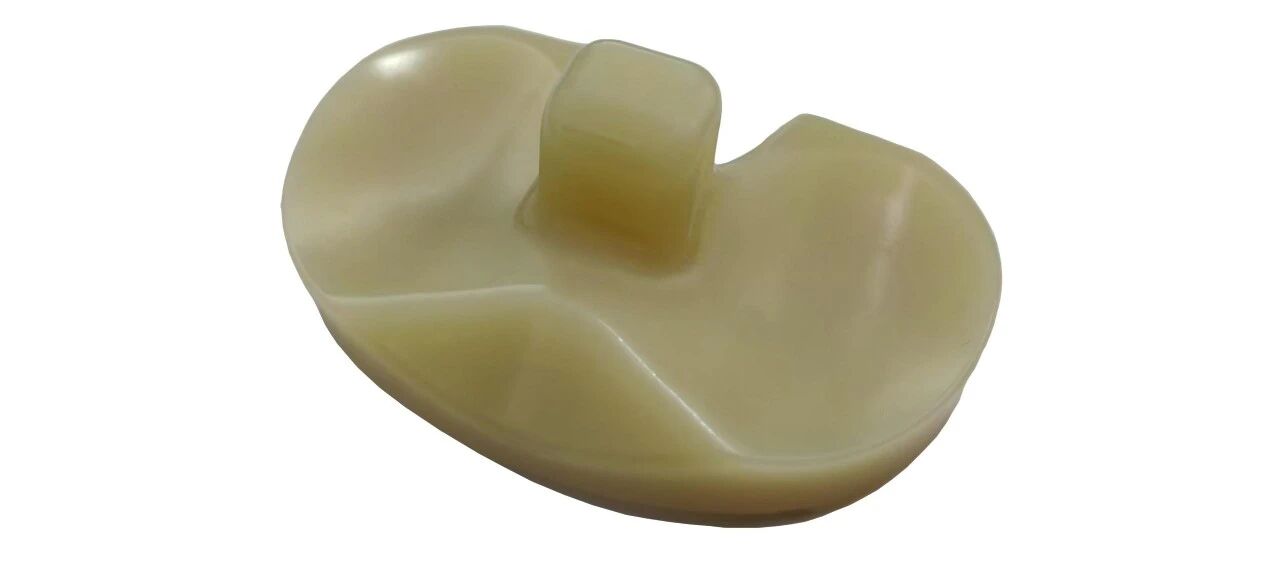
③Tibial plateau pad:
★ The highly cross-linked ultra-high molecular weight polyethylene (UHMWPE) material incorporated with vitamin E achieves synergistic optimization of wear resistance and mechanical properties. It not only enhances the wear resistance of implants, but also improves their antioxidant and impact resistance performance, thereby extending the service life of implants.
★ Provide PS/CR Type Prostheses.
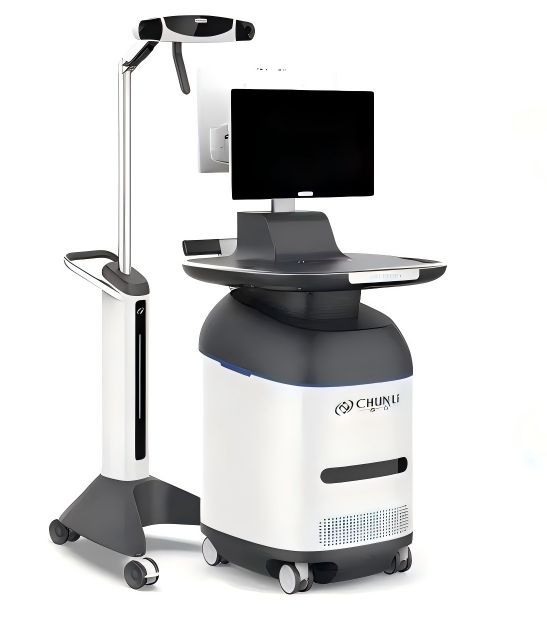
Precision medicine, driving the development of smart orthopedics
Since biotype knee prostheses rely on biological fixation, they impose higher requirements for osteotomy precision. Clover has developed an innovative handheld robotic system for knee surgery, which integrates spatial marker technology and advanced software algorithms to achieve real-time tracking of the position and orientation of surgical instruments. This ensures precise boundary control during surgery, enhances surgical accuracy, and thus enables the system to better serve clinical applications.
Looking Ahead: Improving the Product Lineup of Knee Joint Solutions
With the launch of Clover’s Biotype Total Knee Prosthesis, the company’s comprehensive knee joint solution has been further improved. This not only expands the clinical options for the treatment of knee joint diseases, but also delivers a superior postoperative quality of life for patients. As a leader in China’s domestic joint device industry, Clover Medical will continue to uphold the philosophy of "Absorbing the Essence of Predecessors and Developing High-quality Products for the World", and adhere to the principle of "Developing and Manufacturing Products as if for Personal Use, with Continuous Innovation" in R&D and production. It is committed to providing high-quality products to global customers and emerging as a world-renowned orthopedic device enterprise.
Company Profile
Clover Medical Technology Co., Ltd. (688236.SH; 01858.HK) was founded in 1998. Since its establishment, the company has been committed to advancing the continuous development and research of high-end medical devices. Its product portfolio covers a full range of orthopedic devices, including joint, spinal, sports medicine and trauma products. Meanwhile, the company is actively expanding into such fields as dental care, platelet-rich plasma (PRP) preparation systems and orthopedic surgical robots to continuously broaden its product lines.
Clover Medical holds 160 medical device registration certificates, covering multiple product categories including joints, spine, sports medicine, trauma, dentistry and PRP. The acquisition of multi-category registration certificates has further enriched the company’s product portfolio, expanded its layout in the medical field, continuously met diversified market and clinical needs, and helped enhance its overall competitiveness. While maintaining its leading position in the domestic market, the company is also actively exploring the international market. At present, its products have been exported to more than 50 countries and regions worldwide.



