Great news
In April 2025, the "Biological Total Knee Prosthesis" developed by Beijing Chunli Zhengda Medical Instruments Co., Ltd. was approved for registration by the National Medical Products Administration (NMPA) of the People's Republic of China.
1. Design Background
Total Knee Arthroplasty (TKA) is one of the effective treatment modalities for end-stage knee joint diseases such as osteoarthritis, rheumatoid arthritis, and post-traumatic arthritis. Traditional cemented TKA has long been hailed as the "gold standard" for TKA due to its reliable long-term clinical outcomes and satisfactory prosthesis survival rate. To better meet the needs of younger, obese patients with higher functional and athletic demands, and to improve post-TKA prosthesis survival and patient satisfaction, cementless prostheses were developed 40 years ago. Cementless prostheses aim to reduce the risk of prosthesis loosening through excellent biocompatibility that induces bone ingrowth. For young patients with sufficient bone mass, cementless TKA provides an approach to avoid bone-cement contact press-fit fixation, a fixation method that may address the long-term issues of osteolysis and prosthesis loosening associated with traditional cemented TKA.In recent years, with advancements and innovations in materials science and prosthesis manufacturing technology, remarkable progress has been achieved in the design of fixation concepts and coating material technologies for the new generation of cementless prostheses. This includes bioactive coatings composed of pure titanium and hydroxyapatite (HA) composites. Incorporating hydroxyapatite into the surface of pure titanium to form a bioactive coating significantly enhances the biocompatibility between the material and human tissues, reduces the body’s immune response and rejection, and enables better integration of the implant with surrounding tissues. Meanwhile, hydroxyapatite can guide bone tissue growth along its surface, promote bone tissue ingrowth and integration onto the implant surface, direct new bone tissue to grow around the implant, accelerate the bone healing process, and improve the bonding strength between the implant and bone tissue.
2. Product Introduction
The biological total knee prosthesis consists of a femoral condyle, a tibial plateau insert, a tibial plateau baseplate, and a patellar component. Among these components, the femoral condyle and tibial plateau baseplate are fabricated from cast cobalt-chromium-molybdenum (CoCrMo) alloy conforming to YY 0117.3, while the tibial plateau insert and patellar component are made of vitamin E-mixed highly cross-linked ultra-high molecular weight polyethylene (HXLPE) complying with ASTM F2695. The inner surfaces of the femoral condyle and tibial plateau baseplate are coated with a dual-layer coating: a pure titanium coating meeting the requirements of ASTM F1580 and a hydroxyapatite (HA) coating in accordance with GB 23101.2.
3. Product Features
1. Composite Coating
The non-articular surfaces of the femoral condyle and tibial plateau baseplate are coated with a Ti+HA composite bioactive coating. The vacuum plasma-sprayed pure titanium coating provides an excellent porous structure that serves as a foundation for bone ingrowth. The atmospheric plasma-sprayed hydroxyapatite layer facilitates osteoblast adhesion, differentiation, and growth, promotes bone tissue ingrowth into the pores, forms mechanical interlocking, thereby achieving biological fixation and enhancing stability between the implant and bone tissue.
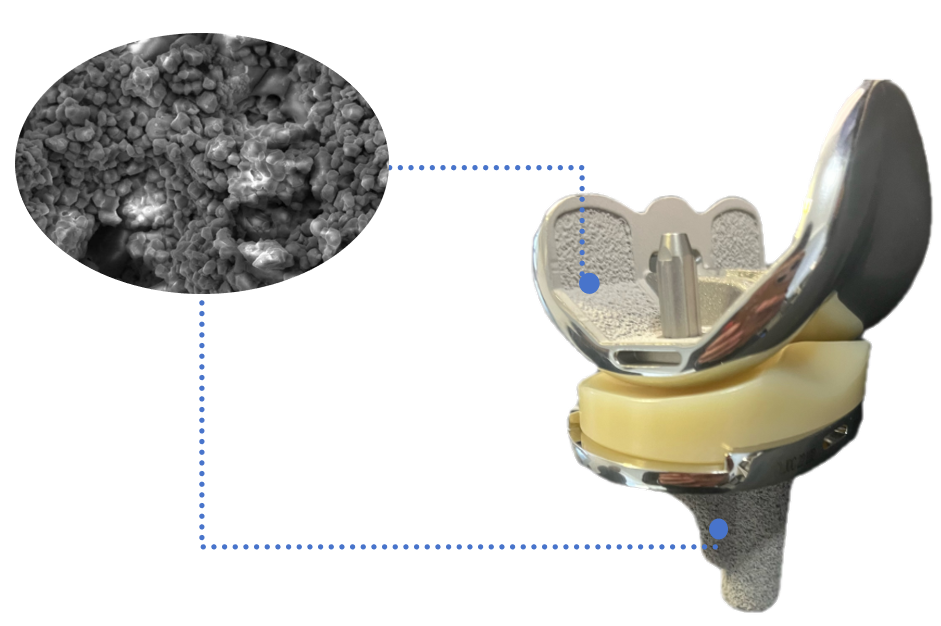
2. Femoral condyle
1.Gradient radius design: Enhances the conformity between the femoral condyles and the insert, and prevents instability during mid-flexion.
2.Thinned anterior condyle with an optimized patellofemoral trochlear design: Reduces patellar crepitus and anterior knee pain.
3.Optimized open intercondylar box design: Minimizes the amount of intercondylar bone resection required.
4.Both PS (Posterior Stabilized) and CR (Cruciate Retention) prosthesis types are available.

3. Tibial Plateau Support
1.Anatomical structure design: Maximizes coverage of the tibial resection surface, enables uniform stress distribution over the proximal tibia, and minimizes irritation of the surrounding soft tissues by the tibial baseplate.
2.Highly polished surface design: The highly polished design of the superior surface of the tibial baseplate reduces wear at the interface between the underside of the insert and the tibial baseplate.

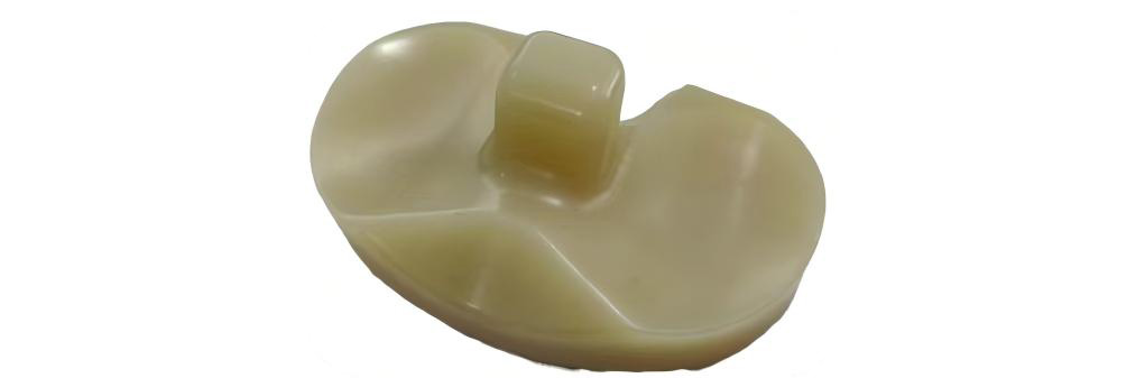
4. Tibial plateau spacer
1.Vitamin E-enhanced highly crosslinked UHMWPE: Balances wear resistance and mechanical properties of polyethylene, improving both wear performance and the implant's antioxidant and impact resistance capabilities.
2.Available in both PS (Posterior Stabilized) and CR (Cruciate Retention) configurations: Offers surgeons flexibility in choosing between posterior cruciate ligament substitution or retention based on patient-specific needs and surgical preferences.
4. Scope of Product Application
It is a cementless prosthesis indicated for total knee arthroplasty (TKA).
5. Company Introduction
Beijing Chunli Zhengda Medical Instruments Co., Ltd. (hereinafter referred to as "Chunli Medical") was founded in 1998, mainly engaged in the R&D, production and sales of medical device products such as orthopedic, dental, biomedical materials and surgical robots. After nearly 30 years of development, Chunli Medical has become the only A+H listed company in the orthopedic and dental fields. It boasts national-level R&D platforms including a National Enterprise Technology Center and a National Postdoctoral Research Station, and holds multiple national-level certifications such as National Champion Enterprise in Manufacturing (Single Product), National High-Tech Enterprise, National "Little Giant" Enterprise (Specialized, Refined, Differential and Innovative), National Intellectual Property Advantage Enterprise, as well as Zhongguancun High-Tech Enterprise.Chunli Medical has led two National Key R&D Programs and is the only domestic enterprise that has widely applied tantalum metal in joint, dental, spinal and sports medicine products. It has also led four "Call for Solutions" projects of the Ministry of Industry and Information Technology, promoting the industrialization of magnesium alloy, hydroxyapatite and tantalum metal and their clinical application in the domestic dental field. Additionally, the company's "Call for Solutions" project on surgical robots was awarded the title of "Outstanding Unit", accelerating the R&D and clinical promotion of domestic surgical robots.
6. Registration Certificate