
Great News
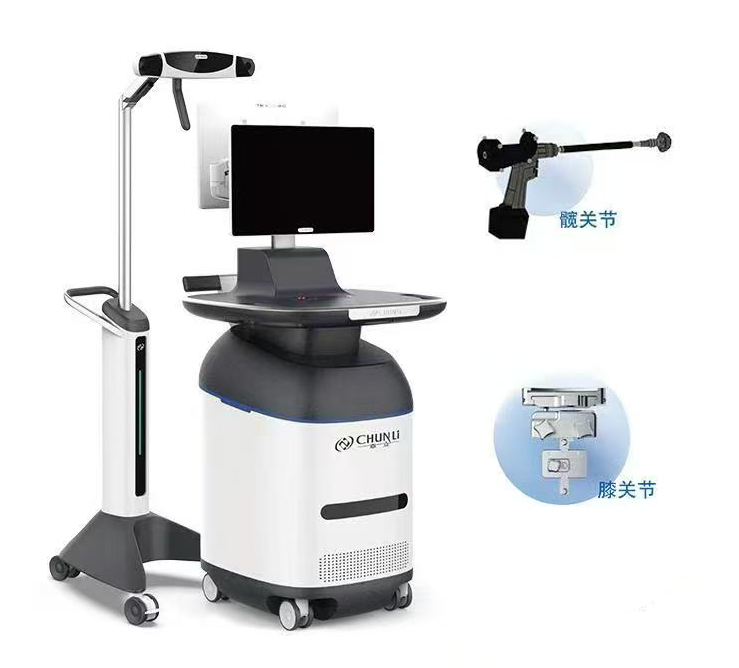
Recently, Beijing Chunli Zhengda Medical Instruments Co., Ltd. (Chunli Medical) had its Hip Arthroplasty Navigation System (Model: Yangtze INS-1) and Knee Arthroplasty Navigation System (Model: Yellow River INS-1) included in the "Beijing 2025 First Batch Catalog of First-of-its-Kind Major Technical Equipment (Medical Health and Other Fields)", specifically in the category of "Medical Health - Medical Devices - Surgical Robots".


Award Background
"First-of-its-Kind (FOIK) Equipment" refers to equipment products that have achieved major technological breakthroughs in China, possess independent intellectual property rights, and have not yet generated market performance in Beijing. This includes the first three units/batches of complete sets of equipment, whole-machine equipment, as well as core components, control systems, basic materials, software systems, and other related products.
Application Requirements - Job Requirements
1)applications for their products to the Office of the Beijing Municipal FOIK Joint Conference through the development and reform departments of each district at any time. The Office of the Beijing Municipal FOIK Joint Conference shall organize and conduct evaluations on a quarterly basis. Products that fail the evaluation shall not reapply within the same year (excluding cases where the application materials are incomplete).
2)All member units of the Joint Conference are requested to coordinate and actively recommend products derived from national or municipal key research projects, guide relevant enterprises and public institutions to formally submit applications through the development and reform commission of the district where the enterprise is registered or the Administrative Committee of Beijing Economic and Technological Development Zone (BDA), and submit recommendation letters to the Office of the Beijing Municipal FOIK Joint Conference.
3)The Administrative Committee of Beijing Economic and Technological Development Zone (BDA) and the development and reform commissions of each district shall organize and guide enterprises and public institutions within their respective jurisdictions to apply for FOIK Equipment, conduct preliminary reviews on the completeness and compliance of the application materials, and form preliminary review opinions and a summary table of collected products.
Product Declaration - Basic Requirements
1)The technical level shall reach an internationally advanced, domestically leading or domestically advanced standard. It shall achieve major breakthroughs in key links such as technology, products, equipment, and processes, occupy a critical position in the industrial chain, and exert a strong driving effect on industrial development.
2)The applicant shall hold clear ownership of independent intellectual property rights in accordance with the law in China.
3)The product shall comply with the relevant national and municipal regulations on product production and sales.
4)The product has not been sold in Beijing, or the sales volume does not exceed 3 units/batches.
Continuously innovate and forge ahead
In recent years, Chunli Medical has focused on the independent R&D of domestic medical devices, actively advancing the technological breakthrough and application of new products, breaking foreign monopolies, and elevating the level of independent innovation in domestic medical devices. Adhering to the R&D and production tenet of "Treat products as if for our own use, and pursue continuous innovation", the company always puts patients first, strives to develop products that meet the highest standards, and undertakes the responsibility and mission of sustainable development in the medical industry.
Taking this as an opportunity, Chunli Medical will continue to deepen technological innovation, actively expand domestic and international markets, and further enhance its core competitiveness. It will forge ahead steadily on the path of promoting industrial development and fulfilling social responsibilities, strive for higher goals, create more brilliant achievements, contribute greater strength to the development of the medical industry in Beijing and even the whole country, and usher in a new chapter belonging to Chunli Medical.
Product Introduction
公司简介
Beijing Chunli Zhengda Medical Instruments Co., Ltd. (referred to as "Chunli Medical") was founded in 1998. It is mainly engaged in the R&D, production and sales of medical device products such as orthopedics, stomatology, biomaterials and surgical robots. After nearly 30 years of development, Chunli Medical has become the only A+H listed enterprise in the orthopedic and stomatological fields.The company boasts national-level R&D platforms including a National Enterprise Technology Center and a National Postdoctoral Research Station. It holds multiple prestigious certifications such as National Manufacturing Single-Item Champion Enterprise, National High-Tech Enterprise, National Specialized, Sophisticated, Unique and New "Little Giant" Enterprise, National Intellectual Property Advantage Enterprise, and Zhongguancun High-Tech Enterprise.Chunli Medical leads two National Key R&D Programs and is the only domestic enterprise that extensively applies tantalum metal in joint, stomatological, spinal and sports medicine products. It has taken the lead in four "Open Bidding for Innovation Tasks (Champion-Bid Program)" projects of the Ministry of Industry and Information Technology, promoting the industrialization of magnesium alloy, hydroxyapatite and tantalum metal as well as their clinical application in the domestic stomatological field. Additionally, the company's surgical robot project under the "Champion-Bid Program" was approved as an "Outstanding Unit", accelerating the R&D and clinical promotion of domestic surgical robots.



