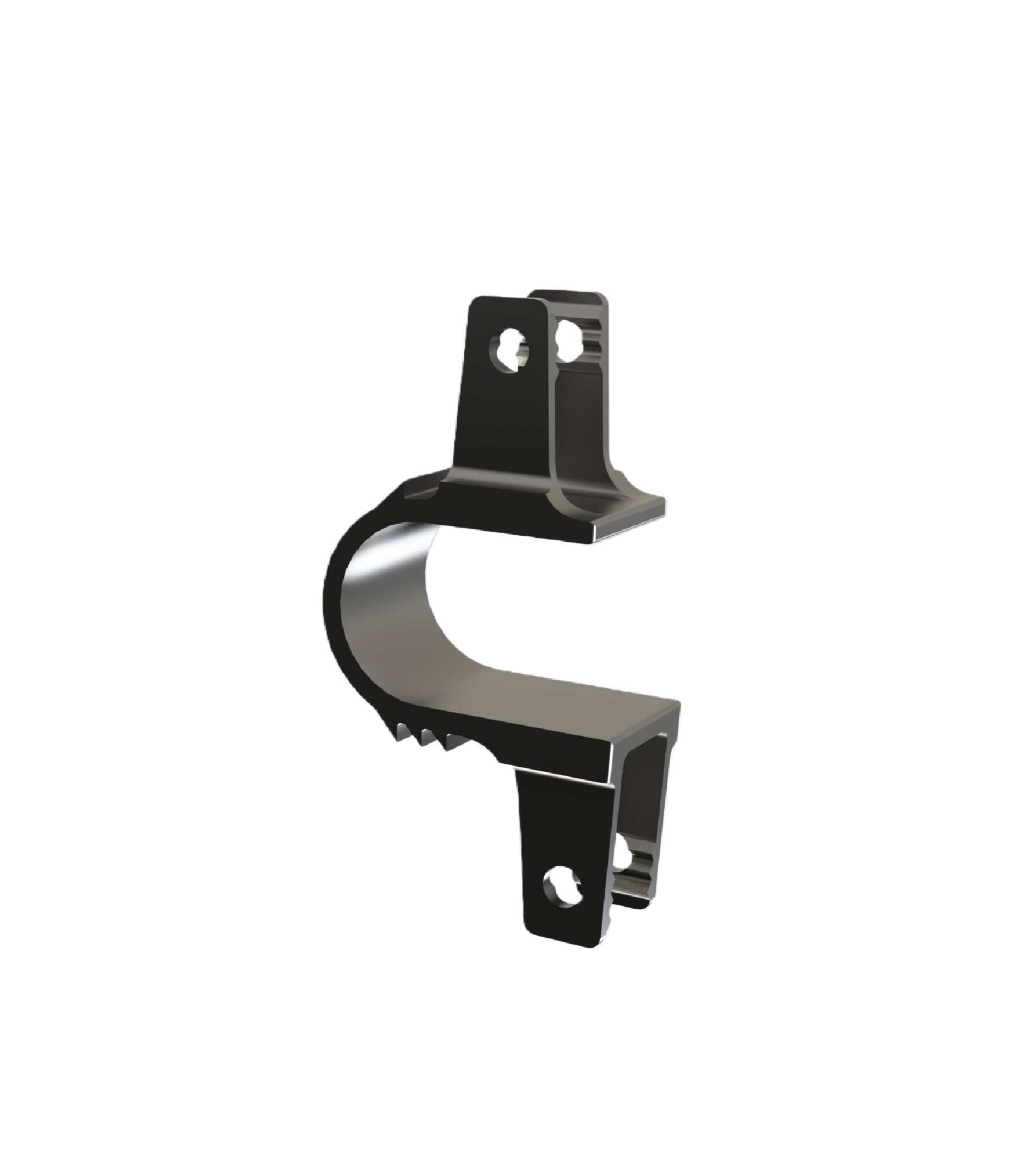
Non-fusion spinous process implant
Non-fusion Spinous Process Implant consists of a U-shaped groove and four sheet-like fixation devices. Manufactured from TC4 titanium alloy per GB/T 13810-2017, with an uncolored surface. Available in sterile (gamma-irradiated, 5-year shelf life) and non-sterile packaging.
Social media