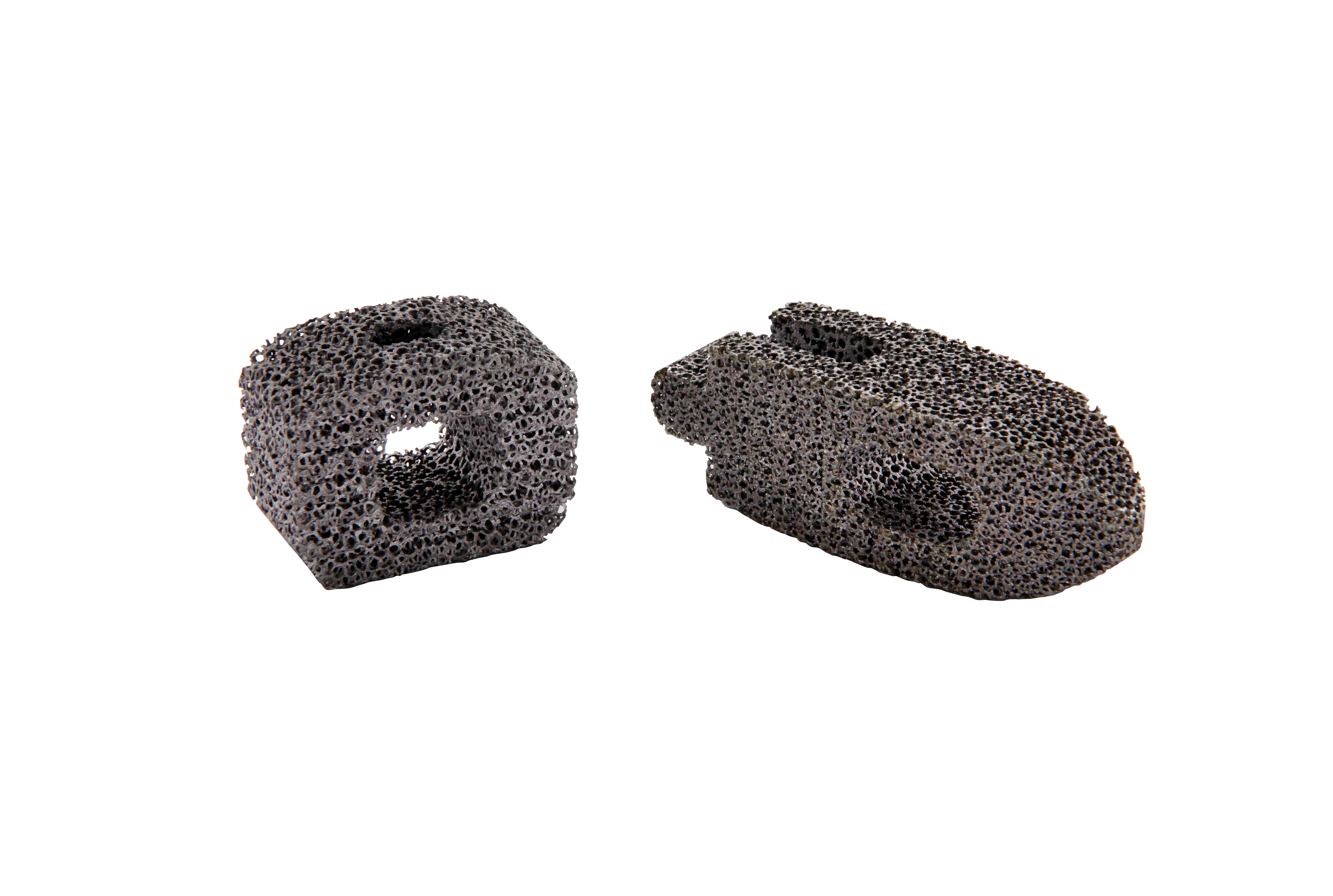
• China's first interbody fusion cage with CVD porous tantalum structure
The pioneering CVD tantalum fusion cage in China, filling the domestic gap.
• Global-leading manufacturing process that breaks monopolies
Produced via world-class CVD (Chemical Vapor Deposition) process—based on chemical gas reaction. Offers better uniformity and stability than 3D printing.
• Porous structure design closer to human bone
Precursor substrate fabrication ensures the final porous structure is more analogous to human bone. Superior toughness to 3D-printed counterparts, with no risk of particle shedding.
• Non-toxic raw material with optimal biological performance
Tantalum material with excellent biocompatibility delivers higher bone fusion rate and superior bone ingrowth.