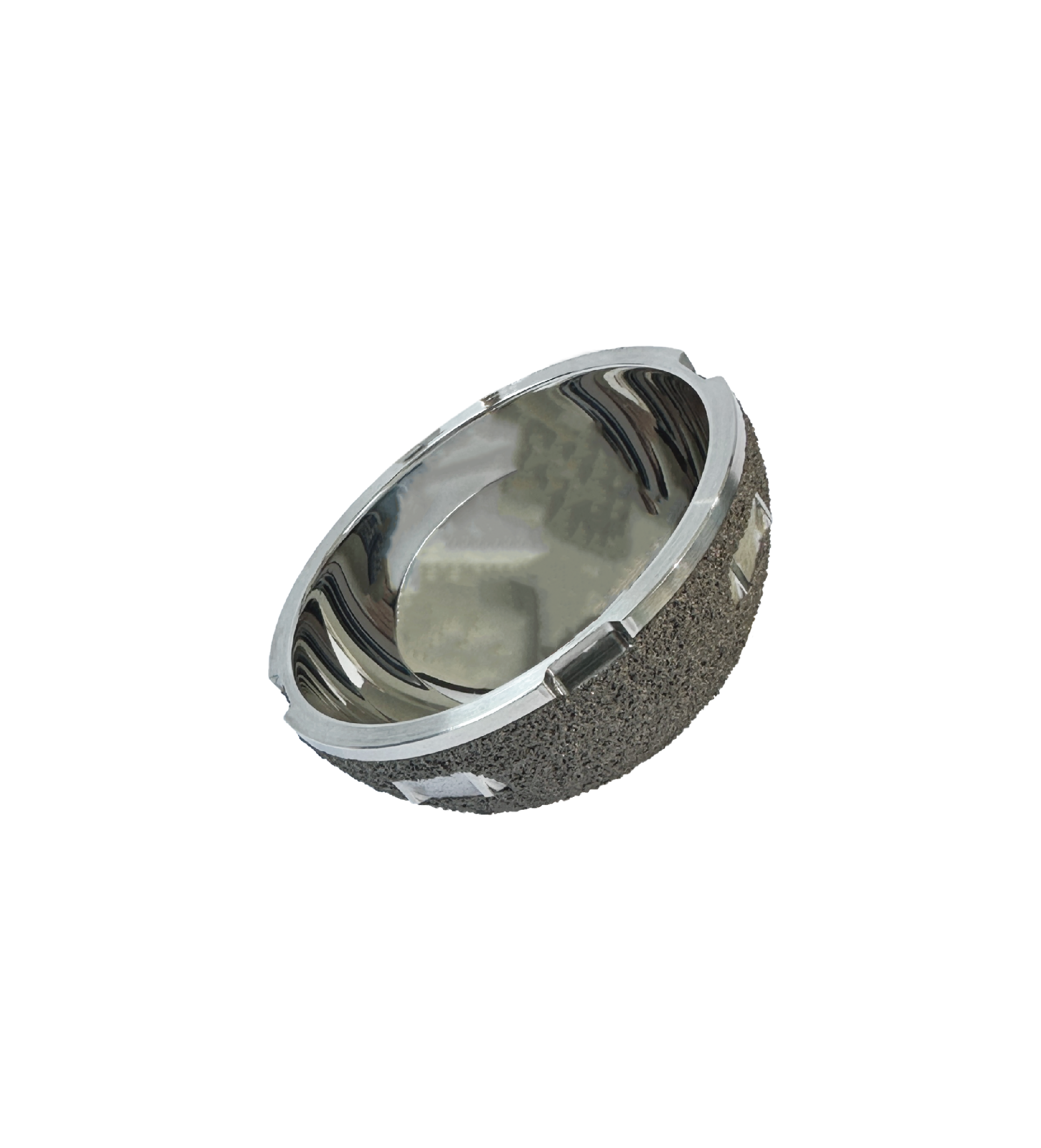
Dual-mobility acetabulum (VE)
This product consists of a metal outer cup and a liner. The metal outer cup is made of cast cobalt-chromium-molybdenum alloy compliant with the requirements of YY 0117.3, and its external surface is coated with titanium alloy that meets the specifications of YY/T 0988.2. The liner is fabricated from vitamin E-containing highly cross-linked ultra-high molecular weight polyethylene. The metal cup is supplied in radiation-sterilized packaging, while the liner uses ethylene oxide (EO) sterilized packaging, with a 5-year sterilization validity period for both components.
Social media