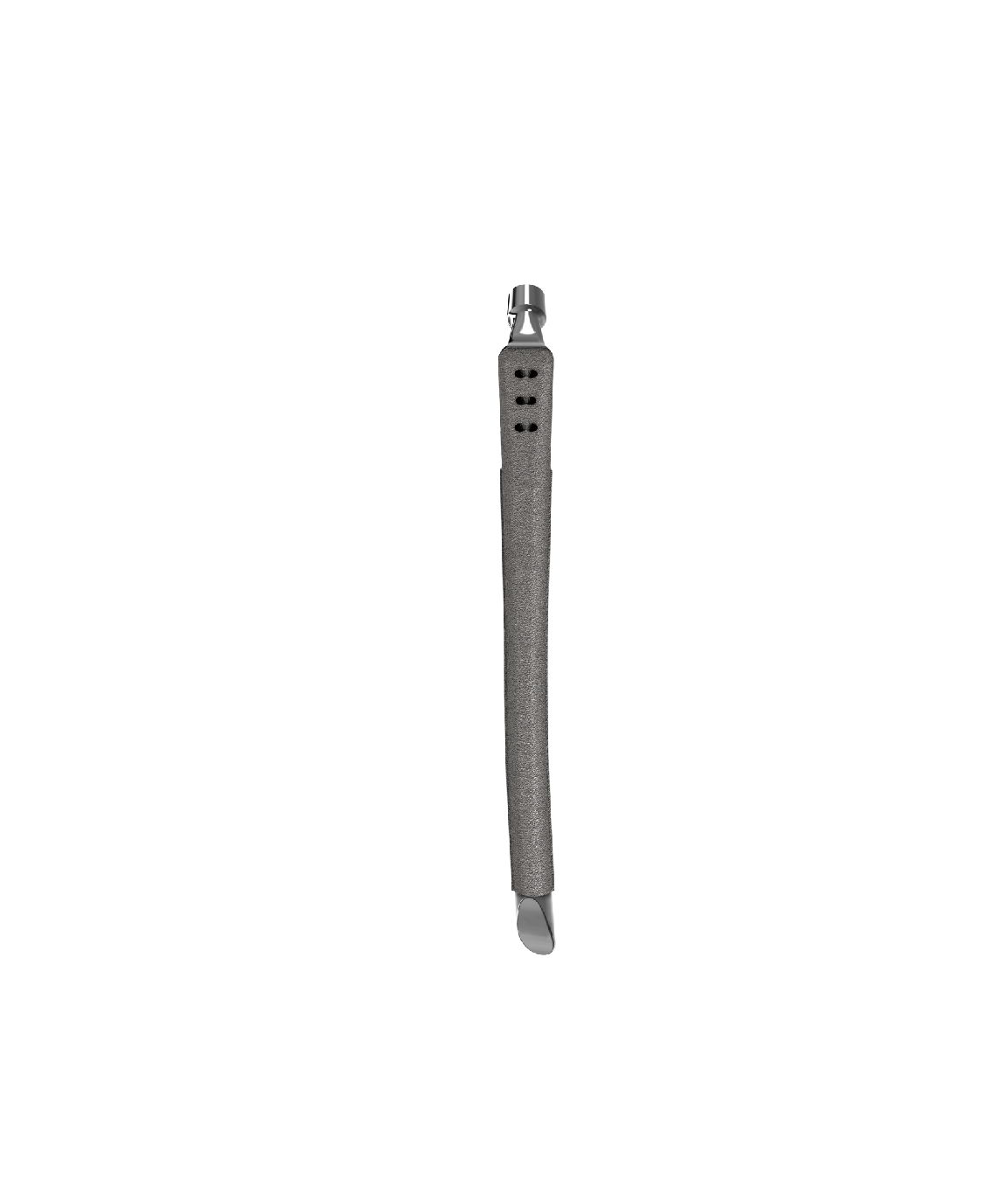
Hip Prosthesis - Revision of Femoral Stem
This product consists of a femoral stem substrate and a surface coating. The femoral stem substrate is made of titanium alloy compliant with the requirements of YY 0117.1, while the surface coating uses pure titanium material meeting the specifications of ASTM F1580. The product is supplied in radiation-sterilized packaging with a 5-year sterile validity period.
Social media