Spinal Posterior Approach Internal Fixation System (Minimally Invasive)
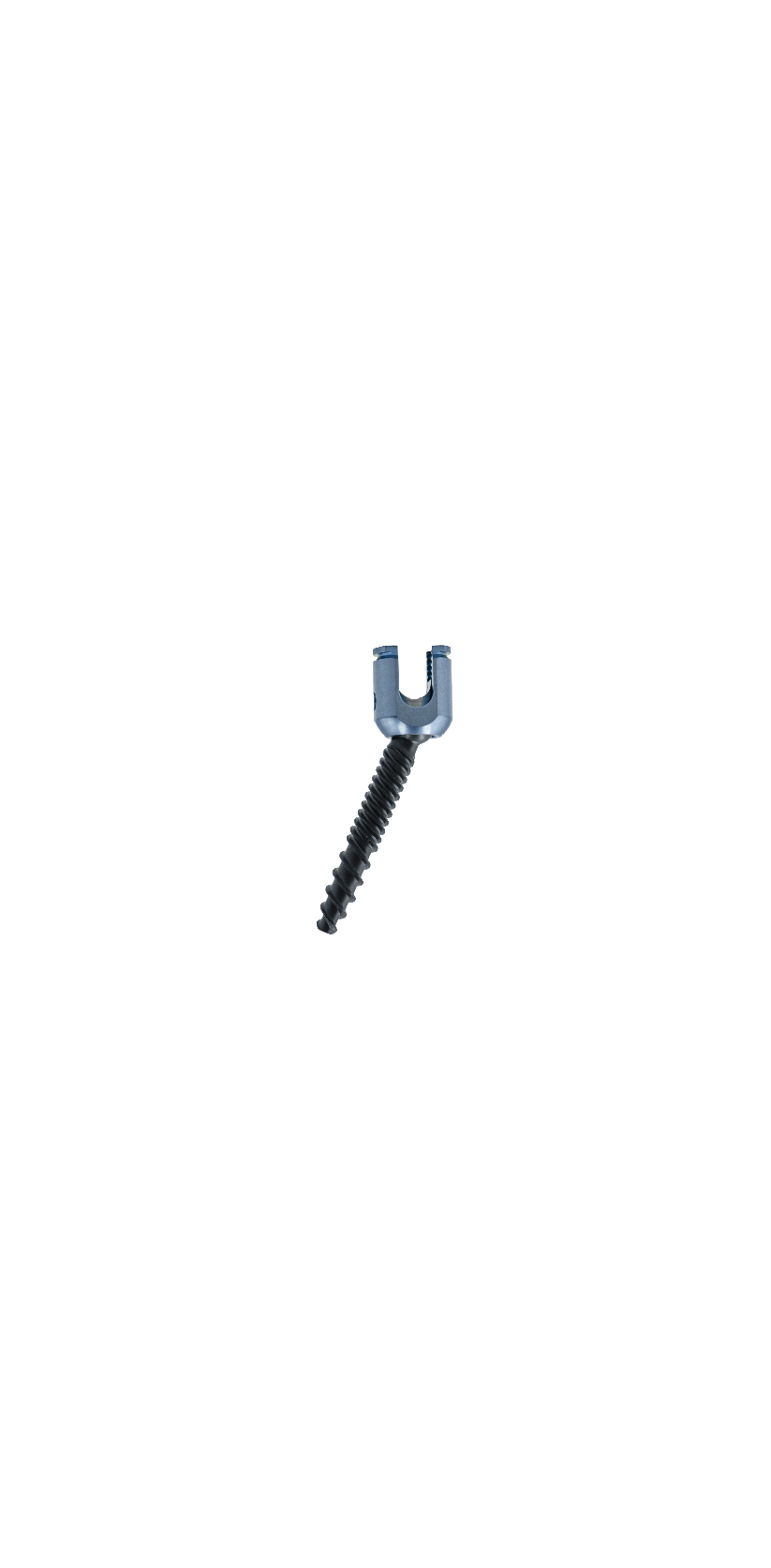
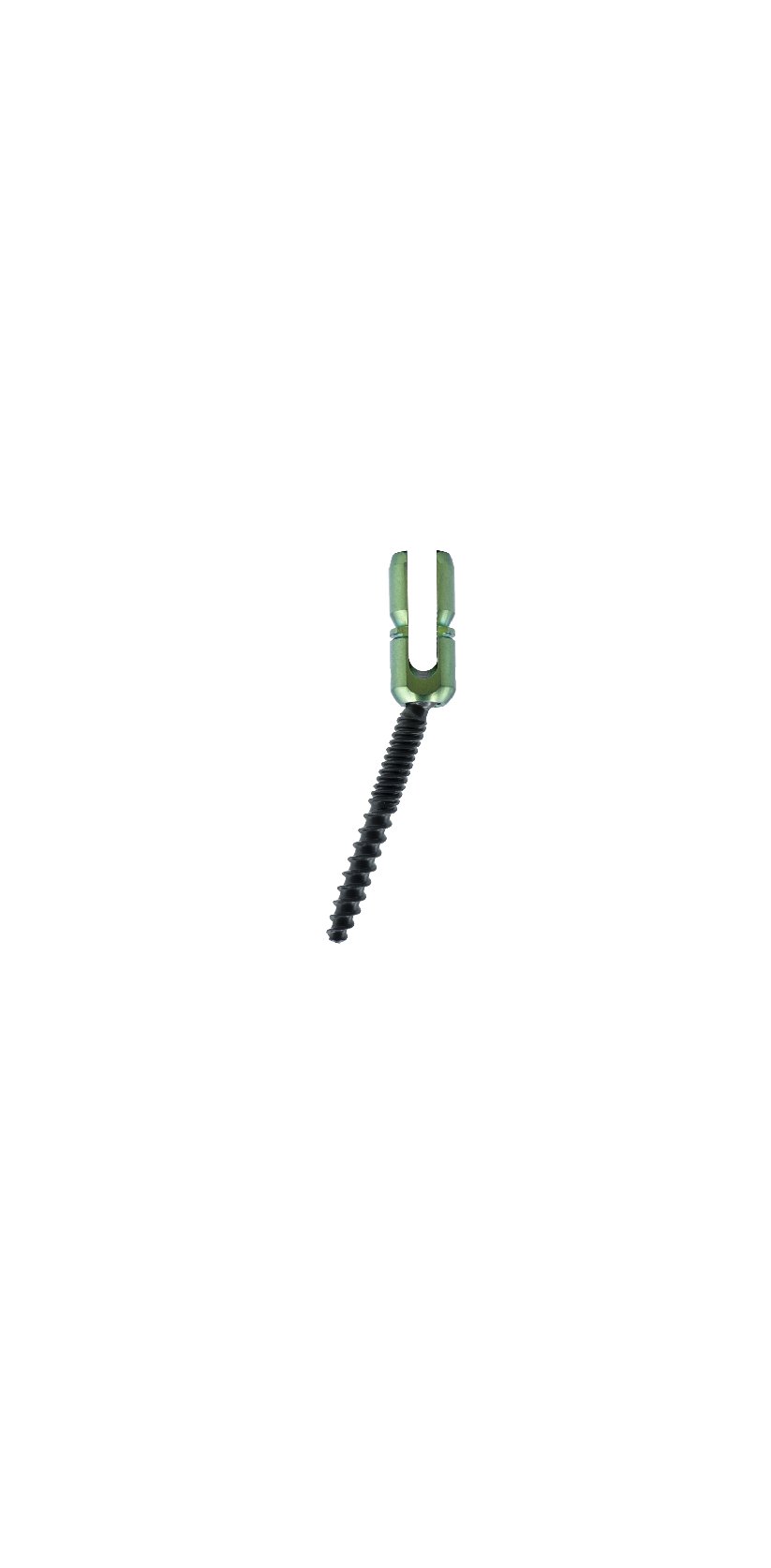
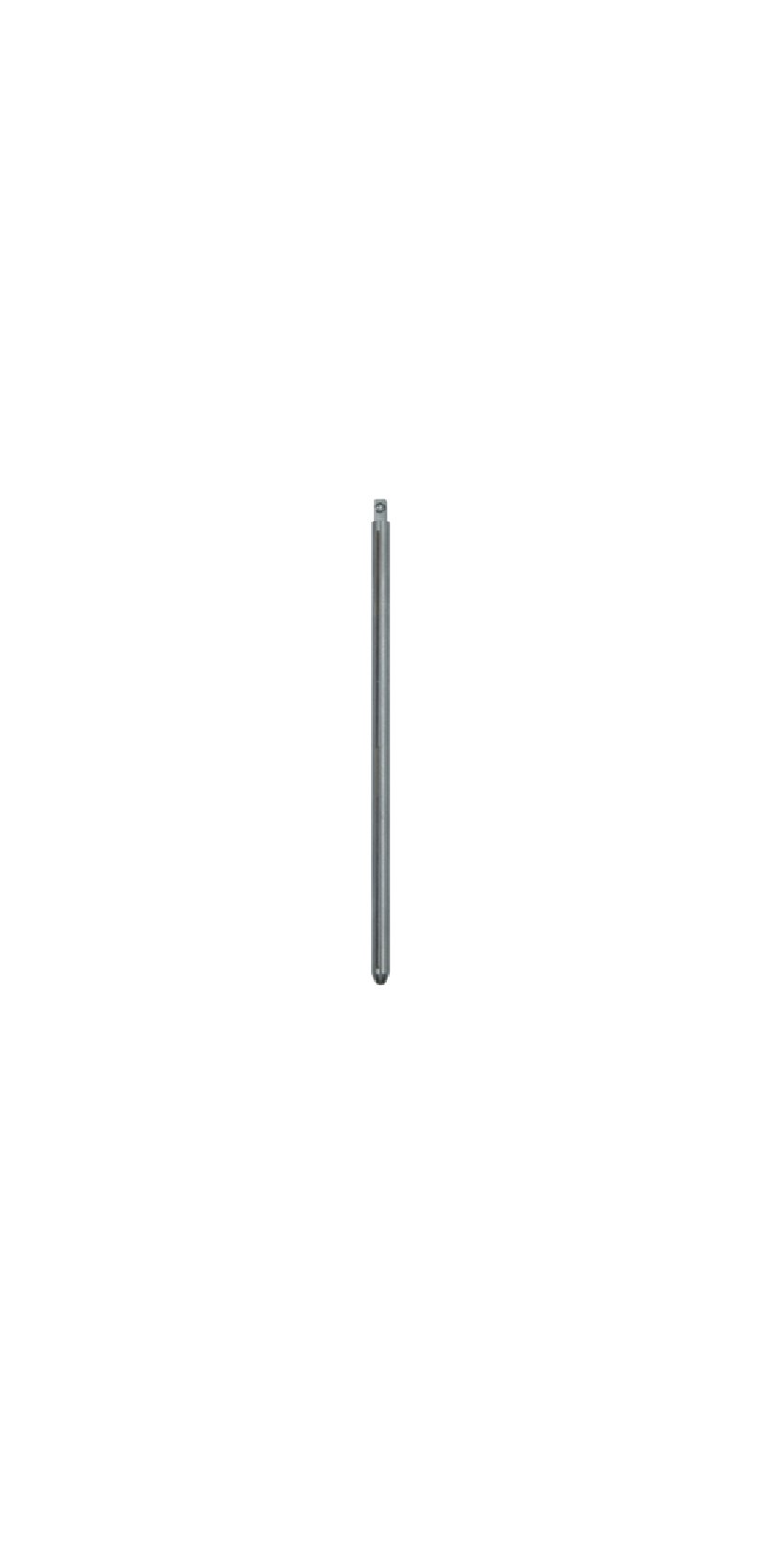
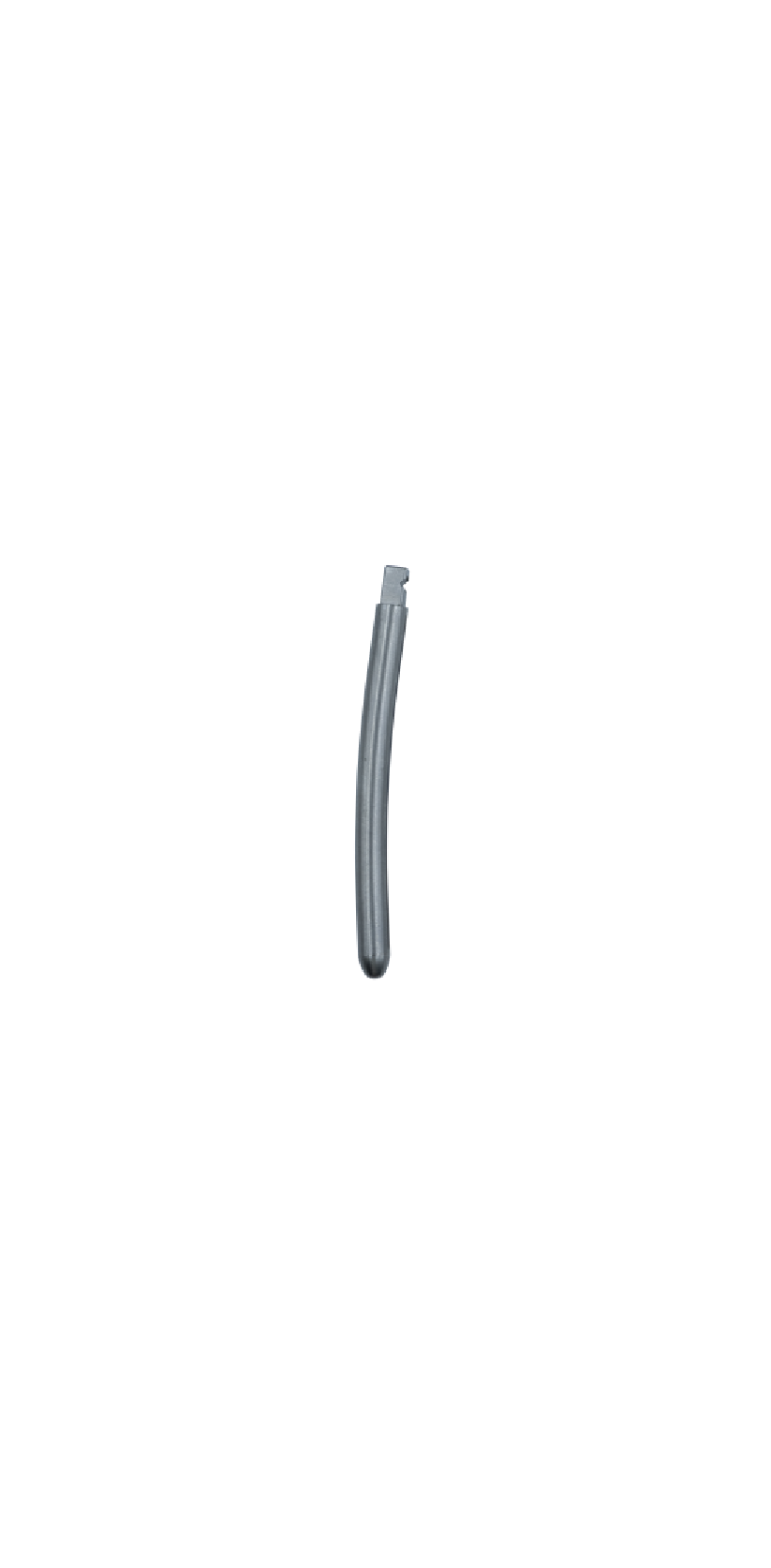
The Spinal Posterior Approach Internal Fixation System (Minimally Invasive) consists of minimally invasive screws, rods, and setscrews. All components of the system are fabricated from titanium alloy (TC4ELI) that conforms to the requirements specified in GB/T 13810. The product is available in two packaging forms: sterilized and non-sterilized; the sterilized packaging adopts radiation sterilization and has a sterile shelf life of 5 years.
Social media