Spinal Lamina Fixation Plate System
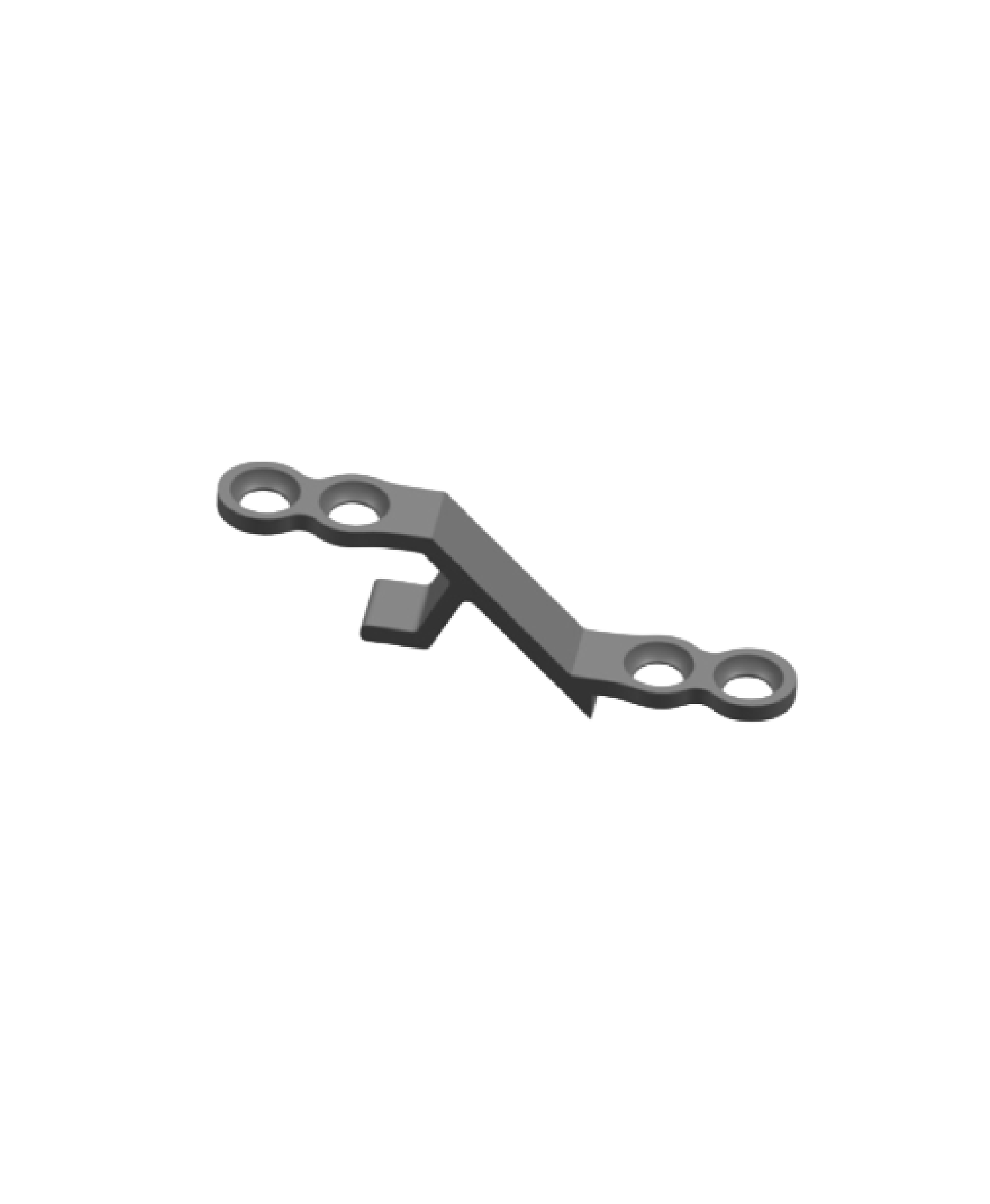
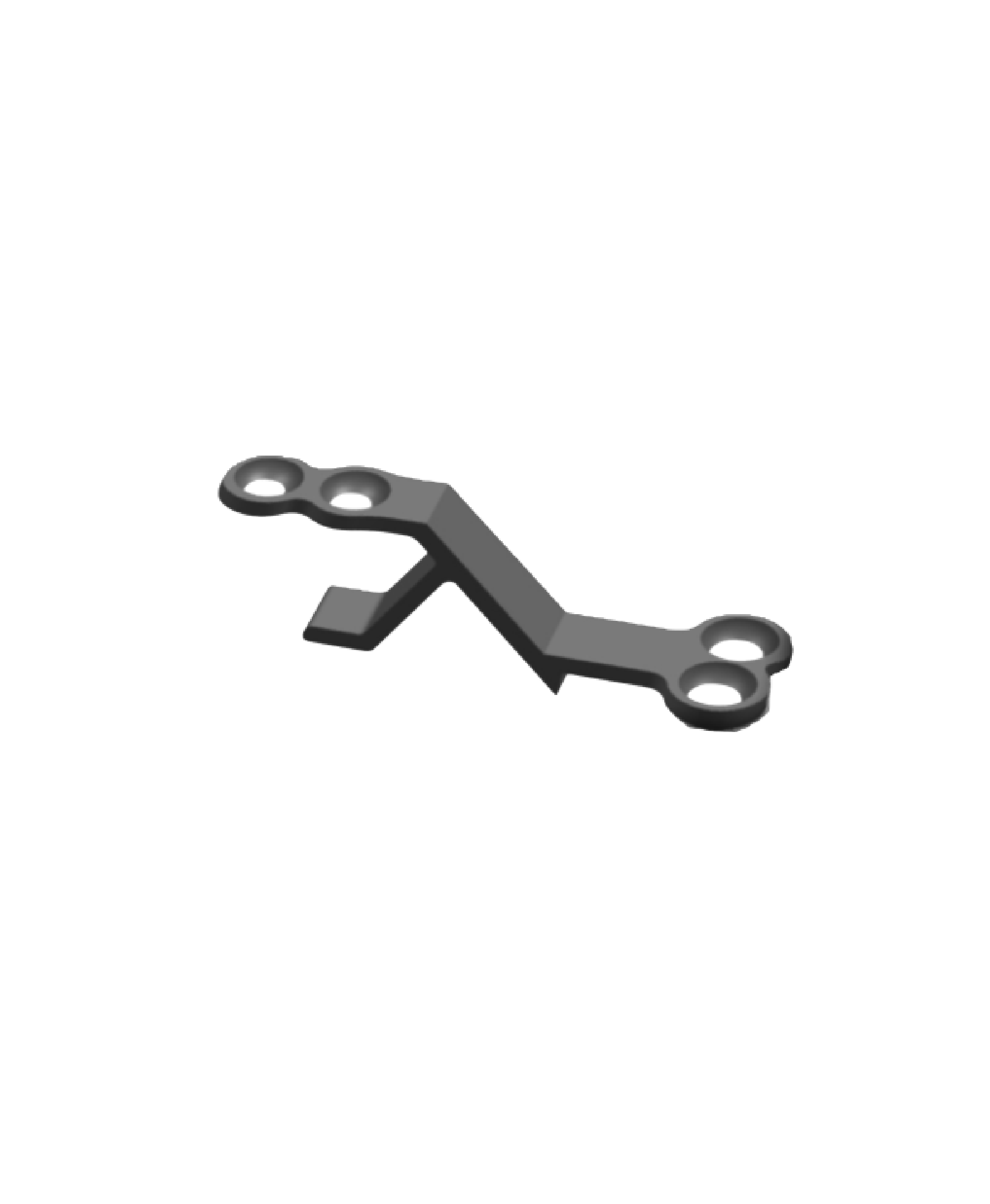
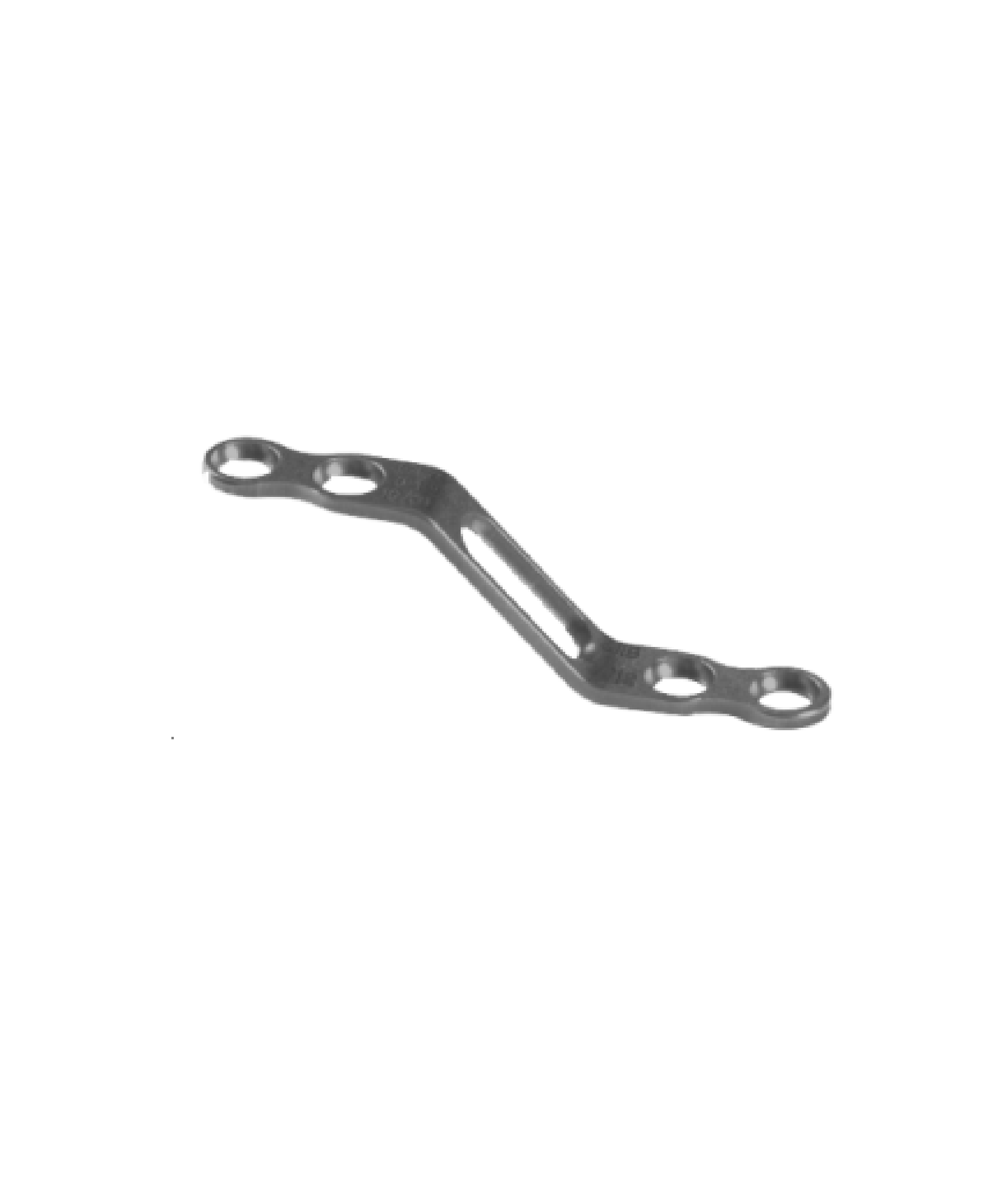
The Spinal Lamina Fixation Plate System consists of fixation plates (Models: ZBI-A, ZBI-B, ZBI-C, ZBI-D, ZBI-E, ZBI-F, ZBI-G) and screws (Model: ZBⅡ). Specifically, the fixation plates are fabricated from TA3G pure titanium that conforms to the requirements specified in GB/T 13810, while the screws are made of TC4ELI titanium alloy complying with the same standard. The product is supplied in both sterilized and non-sterilized packaging; the sterilized version undergoes radiation sterilization and has a sterile shelf life of 5 years.
Social media