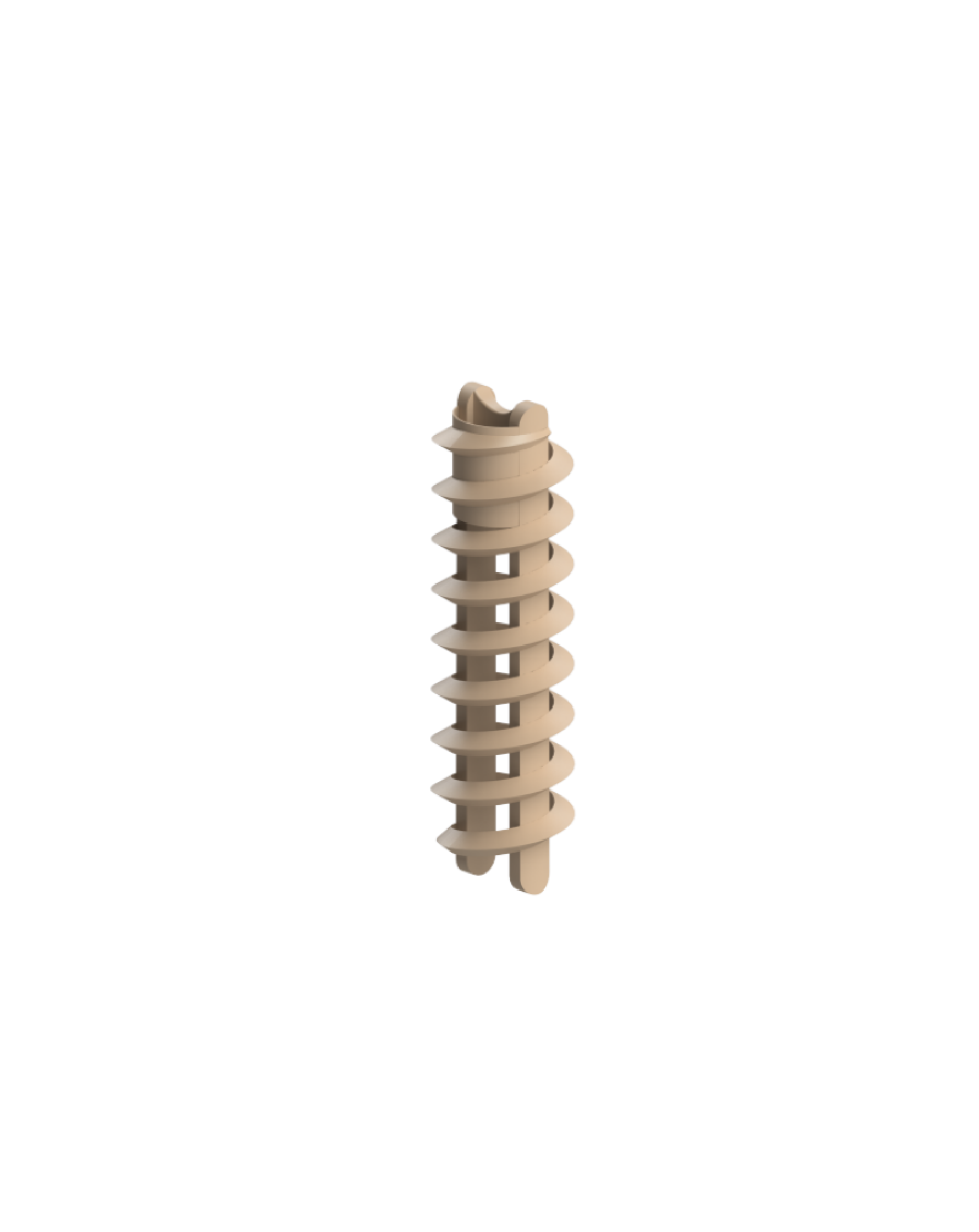
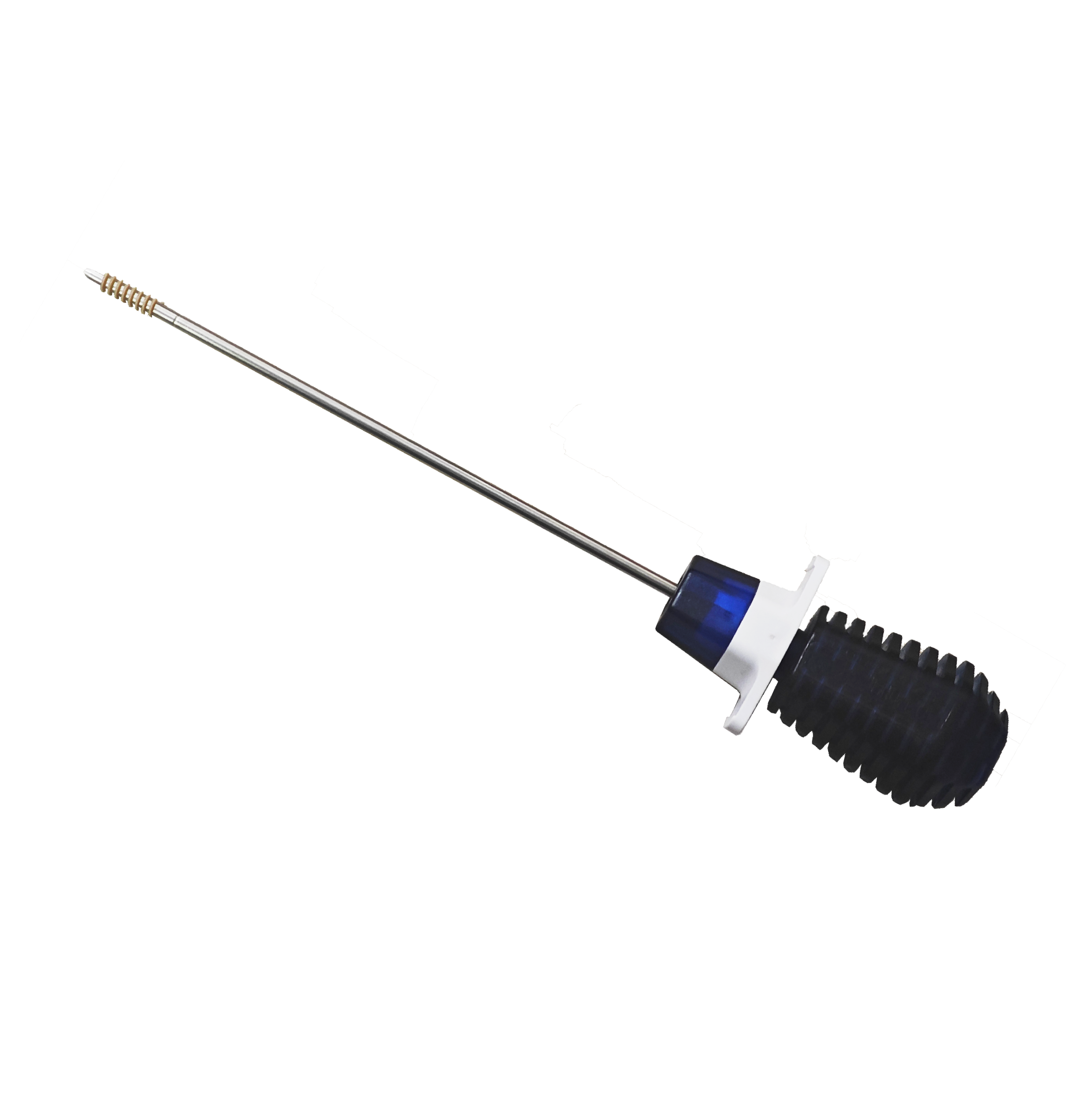
PEEK Suture Anchor
The product consists of an inserter, an anchor, and a suture, and the inserter consists of a handle and a rotary rod. The handle of the inserter is made of PC or ABS material, the material of the handle A is PC, and the material of the handle B and C is ABS. The inserter rod is made of 304 stainless steel (06Cr19Ni10) specified in GB/T 1220; Anchors are made of polyetheretherketone (OPTIMA LT3) material conforming to YY/T 0660 standard. The sutures were braided sutures made of ultra-high molecular weight polyethylene (UHMWPE) conforming to YY/T 1431 standard, and the blue dye was copper phthalocyanine. The product is sterilized by ethylene oxide and valid for 5 years.
Social media