
Great News
Beijing Chunlizhengda Medical Devices Co., Ltd.'s Spinal Titanium Cable has obtained registration approval from the National Medical Products Administration (NMPA) of the People's Republic of China
Product Introduction
A spinal titanium cable is a high-strength metal wire or synthetic material designed to be used in conjunction with other spinal internal fixation devices to enhance spinal stability. It plays a crucial role in the treatment of various spinal conditions, including but not limited to spinal fracture repair, spinal deformity correction, and the management of degenerative spinal diseases.
This product is indicated for internal fixation in cervical spine surgery, used for sublaminar and interspinous fixation. It is applicable to segments C1-C7 and can be used alone or in combination with other spinal internal fixation system products of the same system manufactured by the company using titanium materials.


Product Features
1、Excellent elasticity and high strength.
2、Each titanium cable is composed of 49 strands, arranged into 7 bundles with 7 strands per bundle.
3、The single-headed fixation clip features a 20° adjustable range.
Scope of Application
This product is indicated for internal fixation in cervical spine surgery, used for sublaminar and interspinous fixation. It is applicable to segments C1-C7 and can be used alone or in combination with other spinal internal fixation system products of the same system manufactured by the company using titanium materials.
Product Use
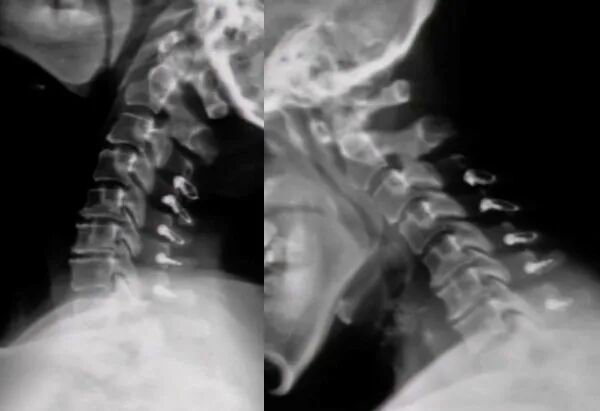
Enhancing Stability:Spinal titanium cables are primarily used to provide additional support and stability to the spine, especially in cases of complex or severe spinal injuries or conditions.
Auxiliary Fixation:They can serve as a supplement to primary fixation devices (such as screws and rods), helping to fixate bony structures more securely.
Promoting Healing:By creating a stable environment, they facilitate better healing of fracture sites or other damaged areas.
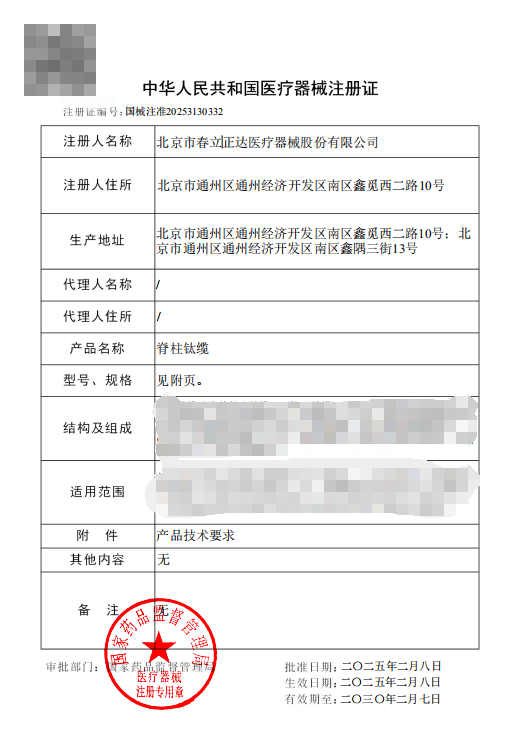
Product Registration Certificate
Company Profile
Beijing Chunlizhengda Medical Instruments Co., Ltd. (abbreviated as “Chunli Medical”) was founded in 1998. It is mainly engaged in the research and development, production, and sales of medical device products such as orthopedic, dental, biomedical materials, and surgical robots. After nearly three decades of development, Chunli Medical has become the only enterprise listed on both the A - share and H - share markets in the orthopedic and dental fields. It boasts national - level R & D platforms including the National Enterprise Technology Center and the National Postdoctoral Research Station. It also holds titles such as National Manufacturing Single Champion Enterprise, National High - tech Enterprise, National Specialized, Sophisticated, Unique, and New “Little Giant” Enterprise, National Intellectual Property Advantage Enterprise, and Zhongguancun High - tech Enterprise. Chunli Medical has taken the lead in two National Key R & D Programs and is the only domestic enterprise that has widely applied tantalum metal in joint, dental, spinal, and sports medicine products. It has led four “Bid - Winning Initiative” projects of the Ministry of Industry and Information Technology, promoting the industrialization of magnesium alloy, hydroxyapatite, and tantalum metal as well as their clinical application in the domestic dental field. Its “Bid - Winning Initiative” project on surgical robots has been approved as an “Outstanding Unit”, accelerating the R & D and clinical promotion of domestic surgical robots



