
Great news
The Non - Fusion Spinous Process Implant developed by Beijing Chunlizhengda Medical Instruments Co., Ltd. has been approved for registration by the National Medical Products Administration (NMPA)
Product Introduction
The non-fusion spinous process implant is primarily used on the spinous processes (the posterior outwardly projecting part of each vertebra). By providing additional support and stability, it helps relieve pain, restore spinal function, and maintain the spine’s natural range of motion as much as possible. Unlike traditional spinal fusion surgery, non-fusion technology aims to resolve pathological issues while maximizing the preservation of spinal mobility. This product consists of a U-shaped groove and four plate-shaped fixation devices, manufactured from TC4 material complying with the requirements of GB/T 13810-2017, with an uncolored surface.

Product Features
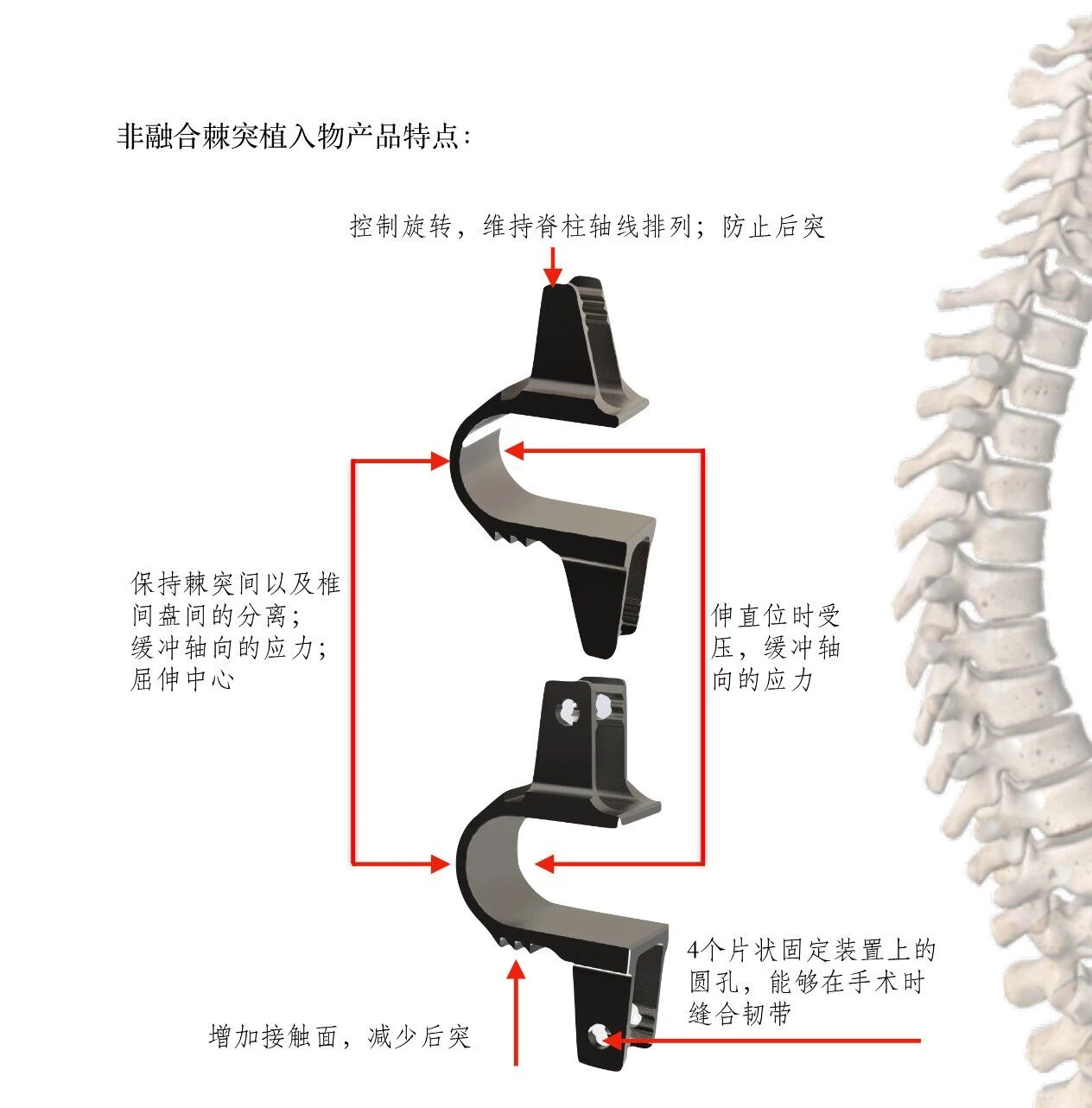
1、Maintain separation between spinous processes and intervertebral discs; cushion axial stress.
2、Control rotation, preserve spinal alignment along the axial direction, and prevent kyphosis.
3、The circular holes on the four plate-shaped fixation devices allow for ligament suture during surgery.
4、Increase the contact surface area to reduce kyphosis.
Scope of Application
This product is indicated for skeletally mature patients with grade 1 or 2 lumbar spinal stenosis between L1 and L5 who have at least moderate functional impairment, accompanied by low back pain, and require surgical decompression.
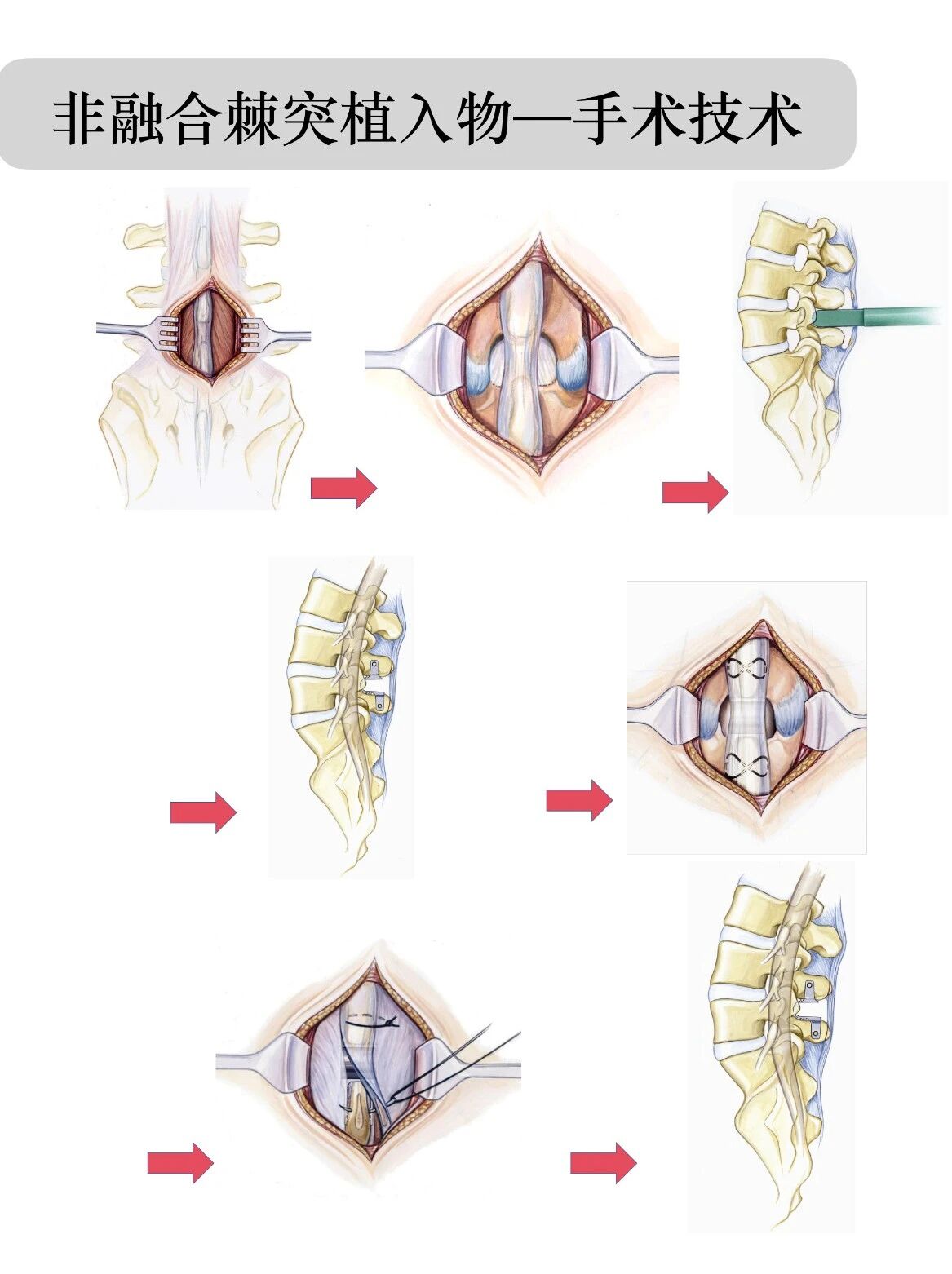
Product operation process
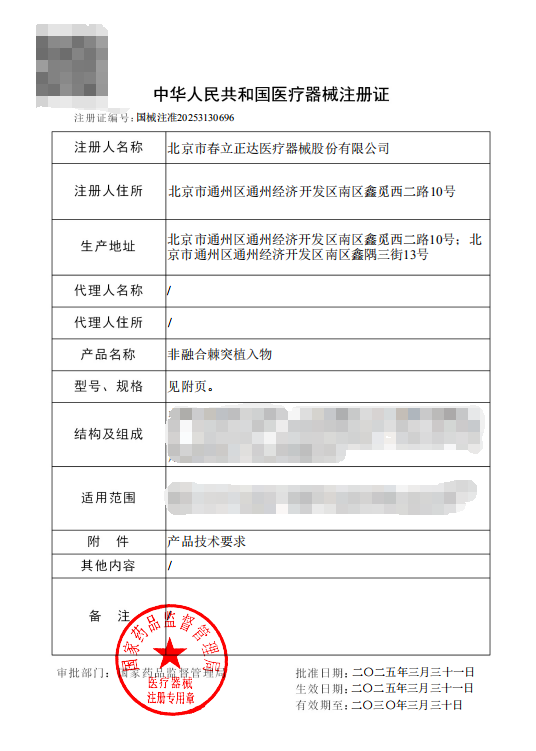
Product Registration Certificate
Company Profile
Beijing Chunlizhengda Medical Instruments Co., Ltd. (hereinafter referred to as "Chunli Medical") was founded in 1998, specializing in the research and development (R&D), production, and sales of medical devices, including orthopedic products, dental products, biomedical materials, and surgical robots. After nearly three decades of development, Chunli Medical has become the only A+H listed company in China's orthopedic and dental fields. It houses national-level R&D platforms such as the National Enterprise Technology Center and the National Postdoctoral Research Station, and holds multiple national-level certifications: National Champion Enterprise in Manufacturing (Single Product), National High-Tech Enterprise, National "Little Giant" Enterprise (Specialized, Refined, Differential, and Innovative), National Intellectual Property Advantage Enterprise, and Zhongguancun High-Tech Enterprise.Chunli Medical has led two National Key R&D Programs and is the only domestic enterprise that has widely applied tantalum metal in joint, dental, spinal, and sports medicine products. It has also spearheaded four "Call for Solutions" projects initiated by the Ministry of Industry and Information Technology (MIIT), advancing the industrialization of magnesium alloy, hydroxyapatite, and tantalum metal as well as their clinical application in China's domestic dental sector. Additionally, the company’s "Call for Solutions" project focused on surgical robots was awarded the title of "Outstanding Unit", accelerating the R&D and clinical promotion of domestic surgical robots.



