
Great News
Approved by the National Medical Products Administration (NMPA), Chunli Medical's dental product Light-cured Flowable Resin has obtained marketing authorization and been officially launched!
Product Introduction
Light-cured flowable resin is an oral restorative material that cures directly intraorally upon irradiation with an external light source. It mainly consists of a resin matrix and inorganic fillers. After exposure to an external light source, the resin matrix undergoes a polymerization reaction to form a cross-linked network structure, which uniformly encapsulates the inorganic fillers and forms a high-strength restoration.
It is indicated for the filling of Class Ⅲ and Class Ⅴ dental caries lesions.
Product Advantages
☛ High Strength: The flexural strength, elastic modulus and compressive strength of the light-cured flowable resin can meet the clinical requirements for the restoration of wedge-shaped defects.
☛Stable Performance: Both the raw materials and packaging materials of the light-cured flowable resin are imported.
☛Complete Range of Common Shades: The available shades of the light-cured flowable resin include A1, A2, A3 and A3.5.
☛Radiopacity: The radiopacity of the light-cured flowable resin is higher than that of an aluminum plate of the same thickness, enabling clinical observation of restoration effects with CT scans.
☛Excellent Flowability & Easy Operation: The light-cured flowable resin features moderate flowability with an optimal marginal filling effect and low polymerization shrinkage. Equipped with injection needles, the product is non-adhesive to dental instruments and easy to shape.
Product operation process
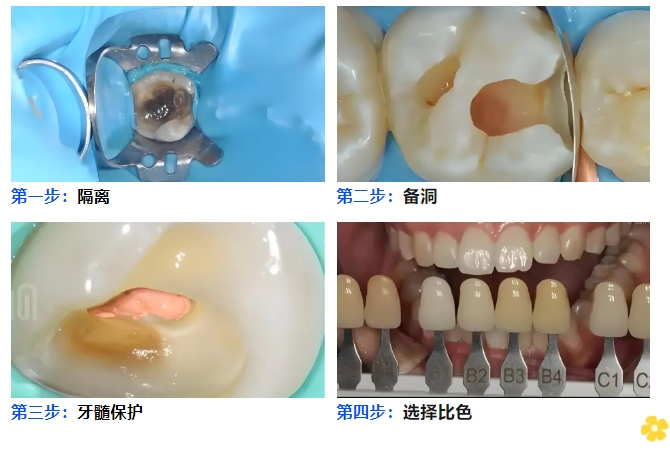
☛Isolation: Rubber dam isolation is recommended.
☛Cavity Preparation: Remove carious dentin and prepare a standard cavity form.
☛Pulp Protection: Apply calcium hydroxide paste for base lining when the cavity is close to the dental pulp.
☛Shade Selection: Select the appropriate shade of the resin.
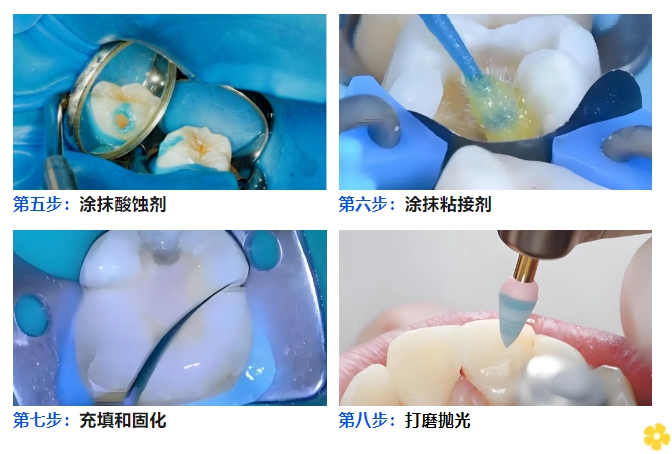
☛Acid Etchant Application: Apply the acid etchant in accordance with its instructions for use, followed by water rinsing and air drying.
☛Adhesive Application: Apply the dental adhesive in accordance with its instructions for use, and perform short-time light irradiation for pre-curing.
☛Filling & Curing: Fill the cavity or defect area with the selected shade of light-cured flowable resin. For a one-step fillable cavity, irradiate with light for 20 seconds for complete curing. If one-step filling is not feasible, adopt the incremental placement technique (applying small amounts multiple times): fill the cavity/defect with a 1-2 mm thick resin layer each time, irradiate for 20 seconds per layer, and repeat the process until the ideal restoration contour is achieved.
☛Finishing & Polishing: Finish and polish the restored tooth surface.
Company Introduction
Beijing Chunli Zhengda Medical Instrument Co., Ltd. (hereinafter referred to as Chunli Medical) was founded in 1998, mainly engaging in the research and development, production and sales of medical device products including orthopedic, dental, biomedical materials and surgical robots. After nearly 30 years of development, Chunli Medical has become the only domestic enterprise listed on both A-share and H-share markets in the orthopedic and dental fields. The Company is equipped with national-level R&D platforms such as the National Enterprise Technology Center and the National Postdoctoral Research Workstation, and holds a portfolio of national-level honors and certifications: it is a National Champion Enterprise in Manufacturing (Single Product), a National High-tech Enterprise, a National Specialized, Sophisticated, Unique and Novel "Little Giant" Enterprise and a National Intellectual Property Advantage Enterprise, as well as a Zhongguancun High-tech Enterprise.Chunli Medical has taken the lead in undertaking two national key R&D programs, and is the only domestic enterprise that has widely applied tantalum metal in dental, joint, spinal and sports medicine products. It has also led four "Project Bidding for Tackling Key Problems" programs launched by the Ministry of Industry and Information Technology (MIIT), which has accelerated the industrialization of magnesium alloy, hydroxyapatite and tantalum metal and their clinical application in the domestic dental field. The Company's surgical robot project under the MIIT's "Project Bidding for Tackling Key Problems" was accredited as an Excellent Unit, which has expedited the R&D and clinical promotion of domestically-developed surgical robots.Adhering to the R&D and production philosophy of "Chunli Dental, Craftsmanship with Ingenuity", Chunli Medical has built a one-stop comprehensive dental solution system, and formulated full-spectrum solutions covering multiple dental sub-sectors including orthodontics, dental implantation, prosthodontics, maxillofacial surgery and surgical robots, emerging as one of the brands with the most complete portfolio of dental product lines in the industry.Upholding the corporate philosophy of "Inheriting the Essence of Predecessors, Developing Fine Products for the World" and the quality policy of "Develop with the Principle of Using Products as Our Own, Pursue Continuous Innovation", Chunli Medical strictly controls the quality of all dental products. Relying on its national-level platforms and national key projects, the Company adheres to the in-depth collaboration of "Industry, Academia, Research, Medicine and Inspection", devotes itself to developing dental products tailored to the anatomical characteristics of the Chinese population, provides high-quality products for global customers, and strives to grow into a world-renowned dental enterprise.



