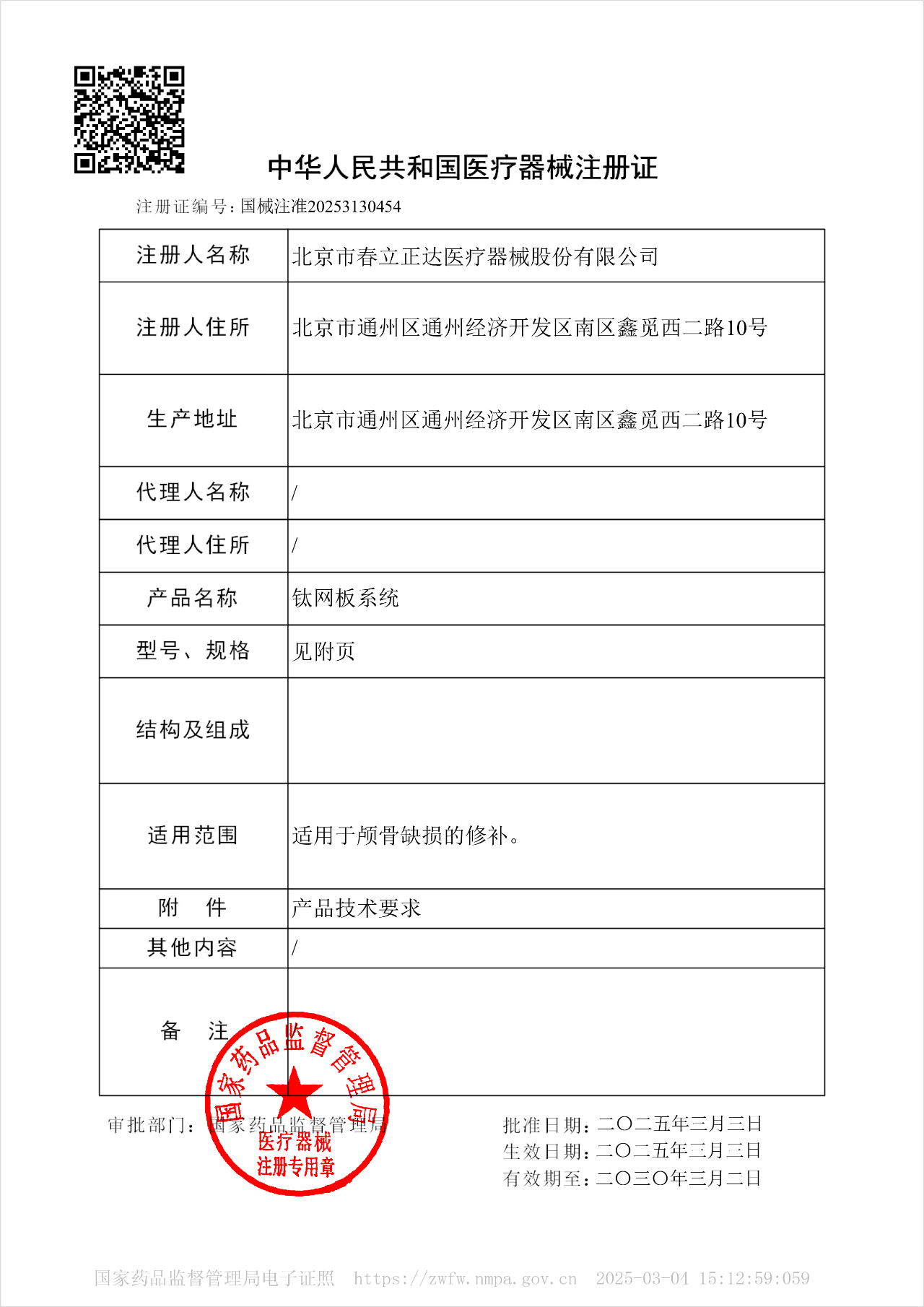
Great News
In March 2025, the "Titanium Mesh Plate System" developed by Beijing Chunli Zhengda Medical Instruments Co., Ltd. was approved for registration by the National Medical Products Administration (NMPA) of the People's Republic of China.
1. Product Introduction
Skull defects caused by trauma, surgery, or other reasons require treatment to restore the integrity and function of the skull. The skull serves as a crucial protective barrier for the brain and facial organs, maintaining the normal operation of the intracranial environment. Traumatic brain injury can cause persistent damage to neurological function, with long-term sequelae including cognitive impairment, psychological disorders, and an increased risk of neurodegenerative diseases.After a skull defect occurs, the complete closure of the cranial cavity is disrupted, leading to instability of the intracranial environment and intracranial pressure. This impairs the circulation of cerebrospinal fluid and blood flow within the skull, hindering the normal functioning of cranial nerve function and thereby triggering a series of skull defect syndrome. Additionally, issues related to cosmetic appearance, safety protection, and psychological trauma may arise.Cranioplasty is a common neurosurgical procedure primarily used to repair skull defects resulting from traumatic brain injury, congenital malformations, craniotomies for cerebral hemorrhage, cerebral infarction, brain tumors, or other causes. It involves repairing the defect area using artificial or autologous materials to restore skull integrity. Titanium alloy is currently the most widely used material for cranioplasty in clinical practice, featuring easy shaping, mild tissue reaction, excellent biocompatibility, high strength and toughness, as well as superior corrosion and wear resistance.The Titanium Mesh Plate System consists of a titanium mesh and fixation screws. The titanium mesh is made of TA3G pure titanium that complies with the requirements of GB/T 13810, while the screws are manufactured from TC4 titanium alloy meeting the specifications of GB/T 13810. For repairing skull defects, cranioplasty with this system not only restores the defect and the patient’s cranial appearance, providing potential protection for the brain against further traumatic injuries, but also effectively restores normal cerebrospinal fluid dynamics and cerebral cortical blood perfusion. This helps reduce intracranial complications and facilitates the recovery of the patient’s neurological function.

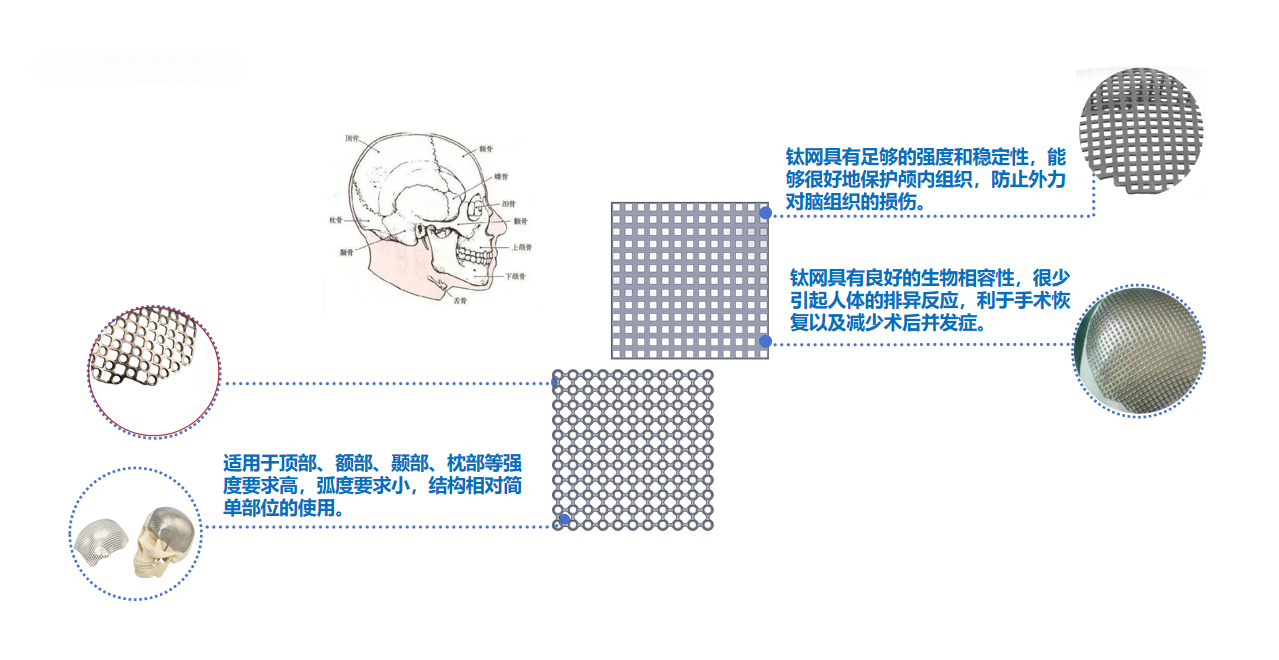
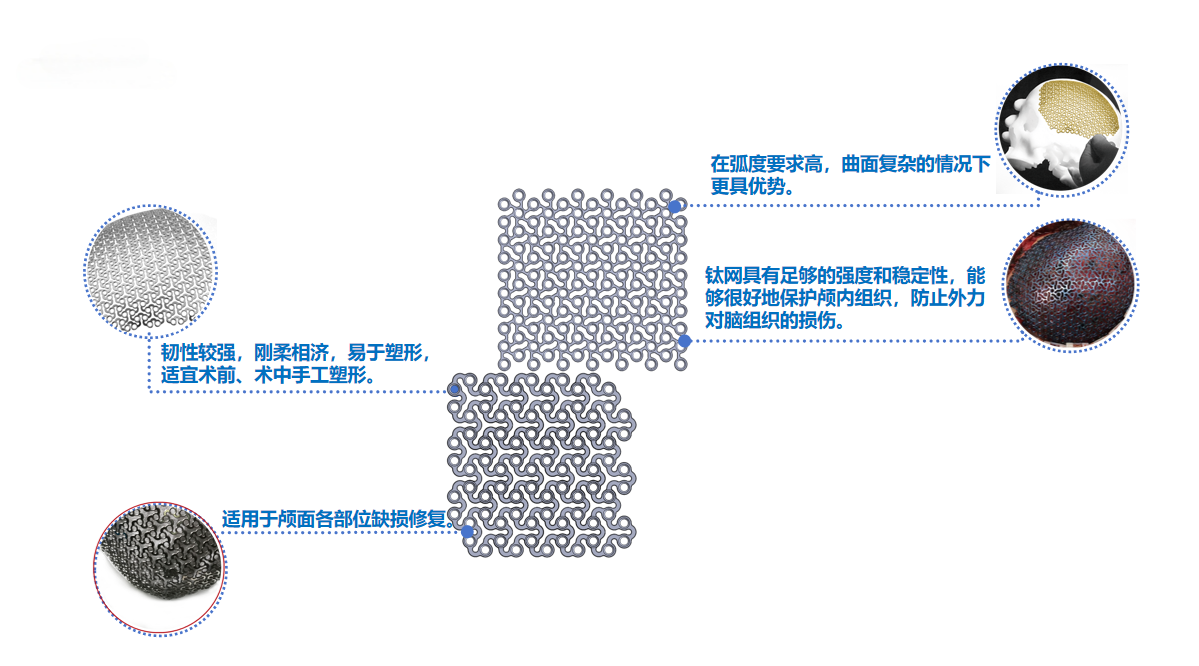
2. Features of Titanium Mesh Products
1.The titanium mesh plate is lightweight, easy to trim, shape, and fix, with a certain degree of flexibility—offering distinct advantages for areas requiring high curvature and complex surfaces.
2.Boasting excellent strength and rigidity, the titanium mesh plate provides stable support to ensure the recovery of fractured or bone-defect sites.
3.Beyond its therapeutic effects, the titanium mesh plate also improves appearance, helping patients restore a natural facial contour. All screw slots feature a countersunk design with a lower profile, enhancing patient comfort.
4.Available in a wide range of models and specifications, the titanium mesh plate includes various patterns such as 2D titanium meshes and 3D titanium meshes, covering all required sizes for skull repair.
5.The titanium mesh plate exhibits superior biocompatibility, enabling good integration with human tissues and promoting tissue repair and regeneration.
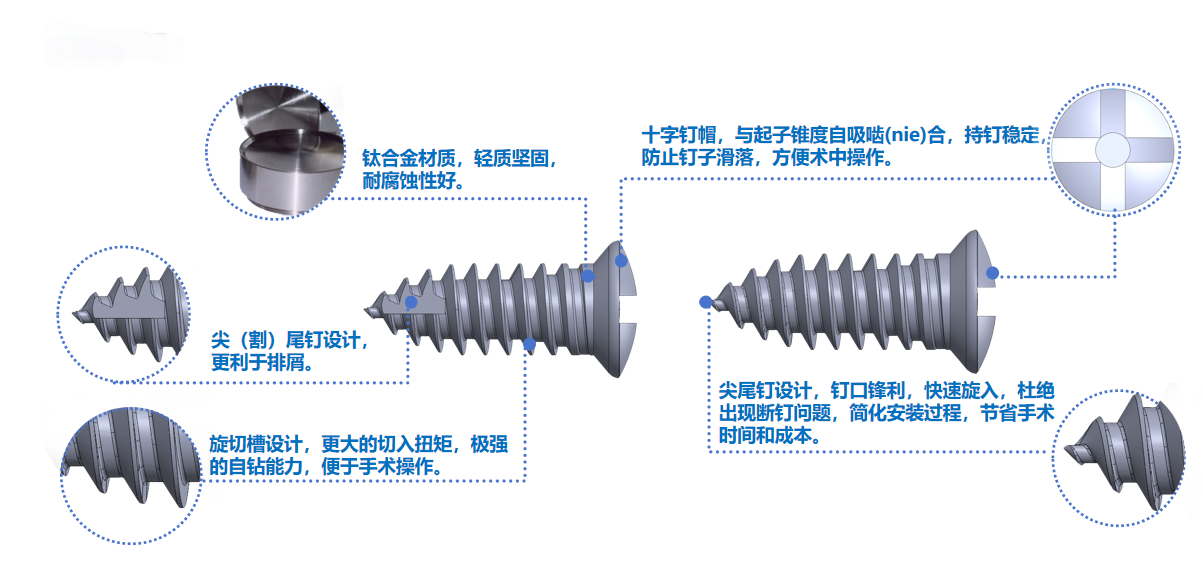
3. Characteristics of Fixed Screws
1.Cross-shaped screw head: Engages with the screwdriver via tapered self-adsorption, ensuring stable nail holding, preventing screw slippage, and facilitating intraoperative operation.
2.Helical cutting groove design: Delivers higher driving torque and exceptional self-drilling capability; no pre-drilling required. The sharp screw tip enables quick insertion, simplifying surgical procedures.
3.Threaded trailing end design of the screw: Minimizes damage to surrounding tissues during insertion, improving surgical success rates.
4.Sharp-tipped and tail-integrated screw design: Eliminates the risk of screw breakage, streamlines the installation process, and saves surgical time and costs.
5.Countersunk design for all screw slots: Features a lower profile, enhancing patient comfort.
6.Comprehensive range of screw models and specifications: Better meets diverse surgical requirements.
4. Scope of Product Application
This product is suitable for repairing cranial defects.
5. Company Introduction
Beijing Chunli Zhengda Medical Instruments Co., Ltd. (hereinafter referred to as "Chunli Medical") was founded in 1998, specializing in the R&D, production, and sales of medical devices such as orthopedic products, dental products, biomedical materials, and surgical robots. After nearly 30 years of development, Chunli Medical has become the only A+H listed company in the orthopedic and dental fields in China. It houses national-level R&D platforms including a National Enterprise Technology Center and a National Postdoctoral Research Station, and holds multiple national-level certifications: National Champion Enterprise in Manufacturing (Single Product), National High-Tech Enterprise, National "Little Giant" Enterprise (Specialized, Refined, Differential, and Innovative), National Intellectual Property Advantage Enterprise, and Zhongguancun High-Tech Enterprise.Chunli Medical has led two National Key R&D Programs and is the only domestic enterprise that has widely applied tantalum metal in joint, dental, spinal, and sports medicine products. It has also spearheaded four "Call for Solutions" projects initiated by the Ministry of Industry and Information Technology (MIIT), promoting the industrialization of magnesium alloy, hydroxyapatite, and tantalum metal as well as their clinical application in China’s domestic dental sector. Additionally, the company’s "Call for Solutions" project on surgical robots was awarded the title of "Outstanding Unit", accelerating the R&D and clinical promotion of domestic surgical robots.
6. Registration Certificate