
Autogenous bone grafting, allogeneic bone grafting and xenogeneic bone grafting are common clinical approaches for bone defect repair. However, their application is limited by problems such as insufficient donor availability, increased surgical trauma and immune rejection. In contrast, synthetic calcium phosphate ceramic repair materials have a physicochemical structure similar to that of natural bone tissue. Their porous micro-nano morphology and surface bioactive ions endow them with excellent osteoconductive and osteoinductive properties, which render them broad application prospects in bone defect repair therapy.
Calcium phosphate ceramics are a category of bioceramics with favorable bioactivity and osteoconductivity, which have garnered extensive attention. Based on differences in calcium-to-phosphorus ratios, calcium phosphate ceramics are classified into three types: hydroxyapatite (HA), tricalcium phosphate (TCP) and biphasic calcium phosphate (BCP).
Hydroxyapatite
Hydroxyapatite (HA) is a naturally mineralized form of calcium apatite with a calcium-to-phosphorus (Ca/P) ratio of 1.67. As the primary inorganic component of human bone, it accounts for approximately 50% of bone mass and exhibits excellent osteoconductive properties. HA boasts the highest stability and lowest solubility among all components of calcium phosphate ceramics. Similar to cancellous bone, HA has relatively low initial mechanical strength and is more vulnerable to tensile and shear forces; however, its compressive strength can reach up to 100 MPa, and it has a higher elastic modulus than natural bone. Despite its favorable mechanical strength and cell adhesion capacity, HA’s poor degradability impedes material resorption and ingrowth of autologous bone.
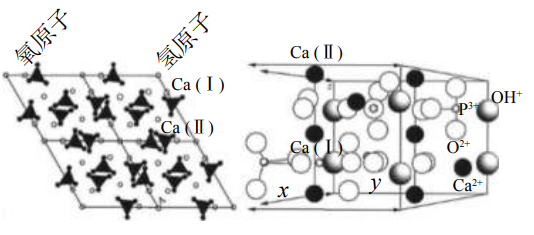
Crystal structure of hydroxyapatite
Tricalcium phosphate
Tricalcium phosphate (TCP) has a calcium-to-phosphorus (Ca/P) ratio of 1.5, which is lower than that of hydroxyapatite (HA), while its water solubility is higher than that of HA. Tricalcium phosphate exists in two crystalline forms: the low-temperature phase (β-TCP) and the high-temperature phase (α-TCP). TCP exhibits favorable osteogenic and degradable properties, yet its degradation rate is difficult to match the rate of new bone formation, and it has relatively poor mechanical strength. Similar to HA, although its porous structure is conducive to neovascularization, TCP lacks initial stability. Studies have confirmed that after implantation, part of the TCP is converted into HA under the influence of multiple factors, which in turn affects the degradation of TCP. Most of the TCP can be phagocytosed and absorbed within 6–24 months, while a small portion can remain in the body for several years.
Biphasic calcium phosphate
Biphasic calcium phosphate (BCP) is composed of hydroxyapatite (HA) with high stability and β-tricalcium phosphate (β-TCP) with higher solubility at varying ratios. The calcium-to-phosphorus (Ca/P) ratio of BCP ranges from 1.5 to 1.67, and its solubility and degradability fall between those of hydroxyapatite and tricalcium phosphate. By adjusting the HA/β-TCP ratio, the material can integrate the advantages of both components—high stability and favorable degradability—thus achieving an optimal degradation rate while facilitating osteointegration. In addition, BCP shares a similar chemical composition with natural bone, making it widely used in clinical applications in stomatology and orthopedics.
Osteoinductive Properties of Calcium Phosphate Ceramic Materials
Early studies suggested that calcium phosphate materials only possessed osteoconductivity. Wells first discovered that calcium salts could promote osteogenesis in 1911. Urist formally proposed the phenomenon of osteoinduction in 1965, and identified that bone morphogenetic proteins possessed osteoinductive capacity to induce ectopic osteogenesis simultaneously. Subsequently, numerous experiments have confirmed that pure calcium phosphate materials without exogenous growth factor supplementation also exhibit a certain degree of osteoinductivity.
1、Characteristics of Bone Repair Materials and Their Osteoinductive Properties
The intrinsic osteoinductivity of calcium phosphate ceramics is closely correlated with their multiple material properties. For this reason, researchers have conducted in-depth investigations respectively from the aspects of chemical composition, pore structure, ionic microenvironment, surface roughness, bone-like apatite formation and nanostructures, aiming to enhance the osteoinductivity of artificial bone substitutes. Experimental data have demonstrated that the material structure exerts the most significant influence on osteoinductivity, followed by surface morphology and chemical composition.
2、Porosity and Bone Induction Properties
Experiments have confirmed that dense calcium phosphate ceramics fail to induce osteogenesis, whereas porous calcium phosphate ceramics can remarkably induce osteogenesis in vivo. The fundamental functions of pores are to accommodate cell growth and provide channels for vascular ingrowth, as well as to enable sufficient exchange of oxygen and nutrients.
3、Solubility and Osteoinductive Properties
The solubility of calcium phosphate ceramics affects protein adsorption by modulating the ionic concentration on the material surface. In general, a relatively high solubility of calcium phosphate ceramics implies superior osteoinductivity. Biphasic calcium phosphate can achieve an optimal surface solubility by regulating the mass ratio of hydroxyapatite to β-tricalcium phosphate, thereby optimizing protein adsorption capacity and further enhancing osteoinductive properties. Based on abundant experimental data, the osteoinductivity of calcium phosphate ceramics is ranked as follows: biphasic calcium phosphate > β-tricalcium phosphate > hydroxyapatite > α-tricalcium phosphate. Among different proportions of biphasic calcium phosphate, it has been found that the composite consisting of 30% hydroxyapatite and 70% β-tricalcium phosphate can promote the high expression of bone morphogenetic protein 2.
4、Macroscopic and microscopic porous structures and bone-inducing properties
Relevant experiments have confirmed that porous calcium phosphate ceramics are capable of inducing bone formation, whereas dense calcium phosphate ceramics cannot achieve this effect. The fundamental function of porous scaffold structures is to accommodate ingrowing cells. Moreover, the channels of interconnected pores serve as the core for body fluids, blood vessels and cell culture scaffolds, providing sufficient oxygen and nutrient exchange for cells. In the case of the standalone application of osteoinductive materials, the loaded cells can be induced to differentiate into osteoblasts.
In addition, micropores (pore size < 10 μm) play a crucial role in promoting osteogenesis. The micropores on the walls of macropores not only facilitate the infiltration of body fluids, but the rough surfaces of pore walls are also conducive to cellular attachment and the expression of osteogenic phenotypes.
5、Surface Morphology and Bone-Inducing Properties of Calcium Phosphate Ceramics
The surface morphology of biomaterials is particularly critical to their osteogenic inductive capacity. Numerous studies have explored the effects of material surface morphology on cellular adhesion and differentiation behaviors. McMURRAY et al. fabricated biomaterials with diverse nanoscale surface topographies and found that nanoscale morphologies significantly promoted the specific differentiation of mesenchymal stem cells. Further studies have demonstrated that neither symmetric nor random nanoarrays could induce osteogenic differentiation, whereas disordered nanoarrays were capable of inducing osteogenic differentiation, indicating that the surface morphology of biomaterials modulates bone formation.
6、Nanostructure of Calcium Phosphate Ceramics and Their Bone-Inducing Properties
Bone apatite consists of nanoscale calcium phosphate carbonate crystals. Traditional calcium phosphate ceramics have simulated bone components and porous structures to some degree, yet there are relatively large calcium-phosphorus particles at the microscale, which may reduce the biological performance of porous calcium phosphate ceramics. From a biomimetic perspective, the nanoscale crystal size can improve the biological properties of calcium phosphate ceramics. Osteogenic cells are prone to interact with the nanoscale surfaces of biomaterials, which promotes their adhesion, proliferation and differentiation.
Besides, calcium phosphate nanoceramics are featured with high specific surface area, nanoscale surface topography, and interconnected macropores rich in micropores. These characteristics can effectively activate and regulate cellular activities, and further yield higher osteoconduction and osteoinduction rates in vivo compared with traditional calcium phosphate ceramics.
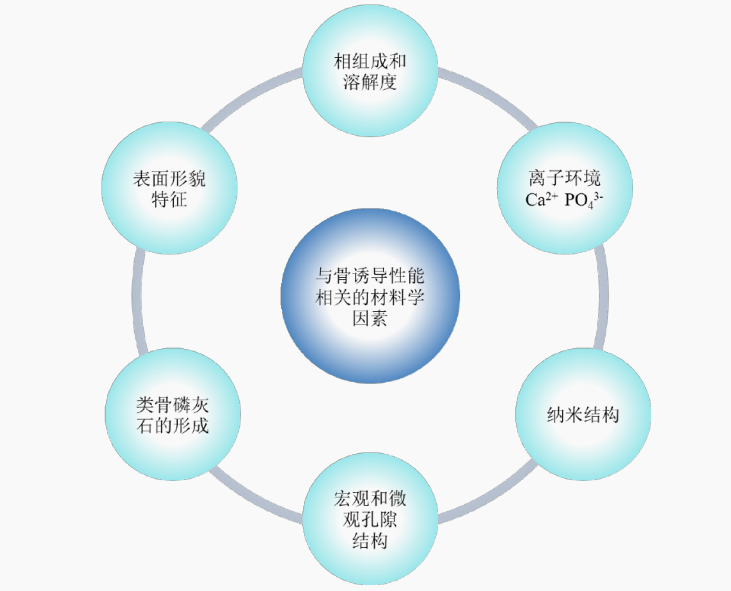
Schematic diagram of material factors related to bone inductive properties
It can be concluded that the calcium phosphate ceramics can be endowed with osteoinductive properties by optimizing the material characteristics including ionic microenvironment, macro-microporous structure, morphological features and nanostructures. These material properties can directly or indirectly regulate the osteogenic induction process. Based on these findings, the osteogenic efficiency of calcium phosphate ceramics can be further improved via the optimization of their physicochemical properties, which contributes to an in-depth and extensive understanding of the osteogenic mechanism of such materials.
Reference: Lu D, Zhang C, Duan RQ, et al. Osteoinductive properties of calcium phosphate ceramic bone repair materials[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7):1103-1109.



